��Ŀ����
4��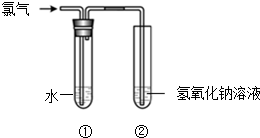 ijͬѧ����ͼ��ʾװ����ȡ��ˮ���������ʵ�飮
ijͬѧ����ͼ��ʾװ����ȡ��ˮ���������ʵ�飮��1��װ�â��з�Ӧ�Ļ�ѧ����ʽ��Cl2+2NaOH=NaCl+NaClO+H2O��װ�â��е�NaOH��Һ��������bcҲ������β�����������ã�����ĸ����
a��NaCl��Һ b��FeSO4��Һ c��Na2SO3��Һ
��2��ʵ�����һ��ʱ���װ�â�����Һ�ʻ���ɫ���ɴ�˵������Һ��һ�����е�������Cl2���ѧʽ����
��3��ȡ����װ�â��е���Һ������ɫʯ����ֽ�ϣ���ֽ�ȱ�����ɫ��˵����ˮ�������ԡ�Ư�ף���ǿ�����ԣ��ԣ�
��4����֪������ͨ���ȵ�����������Һ�У��ɷ������·�Ӧ��Cl2+NaOH��A+NaClO3+H2O��δ��ƽ�����У�A�Ļ�ѧʽ��NaCl��
���� ��1������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ��
��2�������ʻ���ɫ��
��3����ɫʯ��������ɫ�����������Ư���ԣ�
��4������������ԭ��Ӧ���������ȵĻ��ϼ�������Ҳ�н��ͣ�Cl2��NaClO3�ȵĻ��ϼ����ߣ���A���ȵĻ��ϼ۱�0�۵ͣ�����A�е�����-1�ۣ�
��� �⣺��1������������������Һ��Ӧ�����Ȼ��ơ��������ƺ�ˮ����Ӧ����ʽΪ��Cl2+2NaOH=NaCl+NaClO+H2O��NaOH��Һ���Ժ�����֮�䷴Ӧ��FeSO4��Һ��Na2SO3��Һ�ܺ�������Ӧ������β�����������ã�
�ʴ�Ϊ��Cl2+2NaOH=NaCl+NaClO+H2O��bc��
��2��ֻ�������ʻ���ɫ���������ʶ�����ɫ�����Ժ���Cl2���ʴ�Ϊ��Cl2��
��3����ɫʯ��������ɫ�����������Ư���ԣ�ȡ����װ�â��е���Һ������ɫʯ����ֽ�ϣ���ֽ�ȱ�����ɫ��˵����ˮ�������Ժ�Ư���ԣ��ʴ�Ϊ�����ԡ�Ư���ԣ���ǿ�����ԣ���
��4������������ԭ��Ӧ���������ȵĻ��ϼ�������Ҳ�н��ͣ�Cl2��NaClO3�ȵĻ��ϼ����ߣ���A���ȵĻ��ϼ۱�0�۵ͣ�����A�е�����-1�ۣ�����A��NaCl���ʴ�Ϊ��NaCl��
���� ���⿼�����������й����ʣ�֪����ˮ�еijɷּ����ֳɷֵ������ǽⱾ��ؼ���֪���ǽ�����ǿ�����жϷ�������Щ����Ŀ�ѶȲ���

 �̵Ļ������������Ĵ����������ڸɵ��ԭ�������ȣ�
�̵Ļ������������Ĵ����������ڸɵ��ԭ�������ȣ���1��п�̸ɵ�صķ�ӦΪ2MnO2+Zn+2NH4Cl=2MnO��OH��+Zn��NH3��2Cl2��MnO��OH������Ԫ�صĻ��ϼ�Ϊ+3��
��2����ϵ�ػ�ԭ��ķ�Һ������Mn2+��Fe2+��Zn2+�ȣ�����εμ�Na2S��Һ���������ɵij���ΪZnS���ѧʽ����[��֪Ksp��MnS��=1.4��10-15��Ksp��ZnS��=2.9��10 -25��Ksp��FeS��=6.0��10-18]
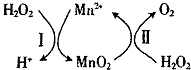
��3��Mn2+��H2O2�ֽ⣺2H2O2��l��=2H2O��l��+O2��g����H1���䷴Ӧ������ͼ��
����֪��Ӧ��ΪMnO2��s��+H2O2��1��+2H+ �� aq��=Mn2+ ��aq��+O2��g��+2H2O��1����H2��д����Ӧ I���Ȼ�ѧ����ʽ���ʱ��á�H1�͡�H2��ʾ����H2O2��1��+Mn2+��aq��=2H+��aq��+MnO2��s����H=��H1-��H2��
��ij�¶�ʱ����10mL0.4mol/L H2O2Һ�е���1��MnSO4�����ֽ⣺2H2O2=2H2O+O2����ò�ͬʱ������O2�������������Ϊ��״���µ�����������
| t/min | 0 | 2 | 4 | 6 |
| V��O2��mL | 0 | 9.9 | 17.2 | 22.4 |
��4���̻������Ǻϳɼ״��������ѵĴ�������֪��
| ��Ӧ | ƽ�ⳣ��KP | |
| 773K | 873K | |
| ��CO2��g��+4H2��g��?CH4��g��+2H2��g�� | 19.4 | 0.803 |
| ��CO2��g��+3H2��g��?CH3OH��g��+H2O��g�� | 6.07��10-9 | 3.65��10-9 |
�ڷ�Ӧ���ƽ�ⳣ������ʽΪK=$\frac{c��C{H}_{3}OH����c��{H}_{2}O��}{c��C{O}_{2}����{c}^{3}��{H}_{2}��}$��
| A�� | ����ˮ��Ӧ��Na+2H2O�TNa++2OH-+H2�� | |
| B�� | �����������O2-+2H+�TH2O | |
| C�� | NaOH��Һ��С�մ���Һ��Ӧ��HCO3-+OH-�TCO32-+H2O | |
| D�� | ����ͨ��ˮ�У�Cl2+H2O?2H++Cl-+ClO- |
| A�� | Y�ļ���̬�⻯����һ�������¿ɱ�Q�������� | |
| B�� | Y�ļ����Ӱ뾶С��Z�ļ����Ӱ뾶 | |
| C�� | Q�ɷֱ���X��Y��Z��W�γɻ�ѧ��������ͬ�Ļ����� | |
| D�� | Z����������Ӧ��ˮ����ֱ���X��Y����������Ӧ��ˮ���ﷴӦ����1molˮʱ���ų���������ͬ |
| A�� | Al����NaOH��Һ��2Al+2H2O+2NaOH�T2NaAlO2+3H2�� | |
| B�� | ʢ��NaOH��Һ���Լ�ƿ�����ò�������SiO2+2OH-�TSiO32-+H2O | |
| C�� | ��FeCl3��Һ�еμӵ��۵⻯����Һ����Һ������2Fe3++2I-�T2Fe2++I2 | |
| D�� | ���ȵ�Fe˿��ˮ�Ӵ��������γ�����ɫ�����㣺2Fe+3H2O��g�� $\frac{\underline{\;\;��\;\;}}{\;}$ Fe2O3+3H2�� |
| ѡ�� | ������ | ������ |
| A | 1-�Ѵ��ķе������ķе��89�� | 1-�Ѵ��������ͨ������������� |
| B | ԭ��ؿɽ���ѧ��ת��Ϊ���� | ԭ�������ӵ�Դ���ܹ��� |
| C | H2SO4�����ӻ����� | ������Һ�ɵ��� |
| D | �Ҷ������KMnO4��Һ������Ӧ | �Ҷ���������� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Ba2+ | B�� | Na+ | C�� | OH- | D�� | Ag+ |
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ����A | O |
| 2 | �� | �� | �� | �� | ||||
| 3 | �� | �� | �� | �� | �� | �� | ⑪ | ⑫ |
��2��д����Ԫ�����������ĵ���ʽ�ͽṹʽ
 ��O=C=O��
��O=C=O����3����ЩԪ�ص����������Ķ�Ӧˮ������HClO4������ǿ��NaOH������ǿ�����γ��������������Ԫ����Al��
��4����Ţܢ�⑪��Ԫ����ɵĻ�����ΪNaClO��д������ʽ
 ��
��