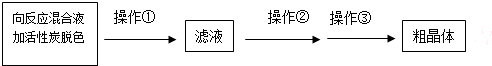
��Ŀ����
15�������������ѷֽ�Ŀ�ʯ֮һ���ӹ�ǰ�轫������ַ��飮����Ҫ�ɷֿɱ�ʾΪFeO•Cr2O3��������SiO2��Al2O3�����ʣ��Ը�����Ϊԭ���Ʊ��ظ���أ�K2Cr2O7���Ĺ�����ͼ��ʾ��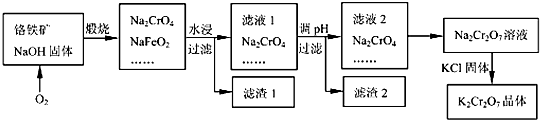
��֪��NaFeO2+2H2O�TNaOH+Fe��OH��3��
��ش�
��1������ǰӦ���������ַ��飬��Ŀ����������������߷�Ӧ���ʣ���Ӧ����֣���������ѧ��ѧ�г��õIJ�������������ʵ�����н��������NaOH�����������գ������¸�ʵ��������ѡ���Ҫ����BCD��
A���մ����� B�������� C�����ż� D��������
��2�����չ�����FeO•Cr2O3������Ӧ�Ļ�ѧ����ʽ��4FeO•Cr2O3+7O2+20NaOH$\frac{\underline{\;����\;}}{\;}$8Na2CrO4+4NaFeO2+10H2O��
��3���Ʊ�����������SiO2��Al2O3���շֱ��ԣ�д��ѧʽ��H2SiO3��Al��OH��3����ʽ����ȥ��
��4�����ɫ����Һ2�м�ϡ���ᣬ�۲쵽��Һ��Ϊ��ɫ�����ϻ�ѧƽ��ԭ���ͱ�Ҫ�����֣��Դ˹��̸��������������2CrO42-+2H+
 Cr2O72-+H2O�Ĵ��ڣ���ϡ����������H+��Ũ�ȣ�ƽ�������ƶ���ʹCrO42-Ũ�ȼ�С��Cr2O72-Ũ��������Һ�ɻ�ɫ��Ϊ��ɫ��
Cr2O72-+H2O�Ĵ��ڣ���ϡ����������H+��Ũ�ȣ�ƽ�������ƶ���ʹCrO42-Ũ�ȼ�С��Cr2O72-Ũ��������Һ�ɻ�ɫ��Ϊ��ɫ����5����Na2Cr2O7��Һ����KC1���壬���K2Cr2O7���������������´��ǣ�����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ����
���� ������ͨ�����գ�����Na2CrO4��NaFeO2��Na2SiO3��NaOH��NaAlO2�Ļ����ϵ��Ȼ���ˮ�ܽ�ù���������������ҺNa2CrO4��Na2SiO3��NaOH��NaAlO2���ٵ�����Һ��pH��ʹƫ��������ȫ��������������ȫ����������������Һ��pHʹCrO42-ת��ΪCr2O72-�������������Һ�м����Ȼ��أ������ܽ�ȼ�С��K2Cr2O7��
��1������Ӵ�����ɼӿ췴Ӧ���ʣ����������ȹ��壬ע���������ƺͶ�������ķ�Ӧ��
��2����������ͼ��֪��Ӧ����FeO•Cr2O3��O2��20NaOH����������Na2CrO4��NaFeO2��10H2O���Դ˿���д��ѧ����ʽ��ע����ƽ��
��3���������衢������������������Һ��Ӧ��������ֱ����ɳ̶ȣ�
��4��CrO42-��Cr2O72-�����ת����Ϊ���淴Ӧ��
��5������Һ�еõ����壬�ɽ��м���Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����Ȳ�����
��� �⣺��1������Ӵ������ӿ췴Ӧ���ʣ����ǰ����������������������Ӵ�����ӿ췴Ӧ���ʣ���Ӧ����֣����չ��������Ϊ�������մ������к���SiO2����NaOH������ѧ��Ӧ������Ӧѡ��������������Ӧ�̶������żܺ��������ϣ���BCD��ȷ��
�ʴ�Ϊ��������������߷�Ӧ���ʣ���Ӧ����֣�BCD��
��2����������ͼ��֪��Ӧ����FeO•Cr2O3��O2��20NaOH����������Na2CrO4��NaFeO2��10H2O����ƽ�ɵû�ѧ����ʽ��4FeO•Cr2O3+7O2+20NaOH$\frac{\underline{\;����\;}}{\;}$8Na2CrO4+4NaFeO2+10H2O��
�ʴ�Ϊ��4FeO•Cr2O3+7O2+20NaOH$\frac{\underline{\;����\;}}{\;}$8Na2CrO4+4NaFeO2+10H2O��
��3�����ڹ����ƺ�ƫ����������Һ�з���ˮ�⣬SiO32-+H2O?HSiO3-+OH-��HSiO3-+H2O?H2SiO3+OH-��AlO2-+2H2O?Al��OH��3+OH-������pH������ˮ��ƽ��������Ӧ�����ƶ�����pH����7��8ʱ��ʹ������ȫˮ�����ɳ�������������H2SiO3 ��Al��OH��3��
�ʴ�Ϊ��H2SiO3��Al��OH��3��
��4��CrO42-Ϊ��ɫ��Cr2O72-Ϊ��ɫ����Һ�д���ƽ�⣺2CrO42-+2H+  Cr2O72-+H2O����ϡ����������H+��Ũ�ȣ�ƽ�������ƶ���ʹCrO42-Ũ�ȼ�С��Cr2O72-Ũ��������Һ�ɻ�ɫ��Ϊ��ɫ��
Cr2O72-+H2O����ϡ����������H+��Ũ�ȣ�ƽ�������ƶ���ʹCrO42-Ũ�ȼ�С��Cr2O72-Ũ��������Һ�ɻ�ɫ��Ϊ��ɫ��
�ʴ�Ϊ������2CrO42-+2H+  Cr2O72-+H2O�Ĵ��ڣ���ϡ����������H+��Ũ�ȣ�ƽ�������ƶ���ʹCrO42-Ũ�ȼ�С��Cr2O72-Ũ��������Һ�ɻ�ɫ��Ϊ��ɫ��
Cr2O72-+H2O�Ĵ��ڣ���ϡ����������H+��Ũ�ȣ�ƽ�������ƶ���ʹCrO42-Ũ�ȼ�С��Cr2O72-Ũ��������Һ�ɻ�ɫ��Ϊ��ɫ��
��5����Na2Cr2O7��Һ�м���KCl���壬����ʱ���ظ����Ƶ��ܽ�����¶ȵ����߶��������Ȼ��ص����¶�Ӱ�첻�����Ի��K2Cr2O7����IJ��������ǣ�����Ũ������ȴ�ᾧ�����ˡ�ϴ�ӡ�����ʴ�Ϊ����ȴ�ᾧ��
���� ���⿼���������Ʊ����̺ͷ����ķ����жϣ�Ϊ�߿��������ͣ�������ѧ���ķ���������ʵ�������Ŀ��飬ע������������ʵ�Ӧ�ã������Ϣ�ķ������⣬���������ע������ͻ������������ǽ���ؼ�����Ŀ�ѶȽϴ�

| A�� | Ũ��Ϊ0.1mol/L | B�� | Ũ��Ϊ1mol/L | C�� | ��NaOH 4g | D�� | ��NaOH 0.1mol |

�����ķ�Ӧ���£�CH3CH2CH2CH2OH $��_{H_{2}SO_{4}����}^{Na_{2}Cr_{2}O_{7}}$CH3CH2CH2CHO
��Ӧ��Ͳ������������б����£�
| �е�/�� | �ܶ�/��g•cm-3�� | ˮ���ܽ��� | |
| ������ | 11.72 | 0.8109 | �� |
| ����ȩ | 75.7 | 0.8017 | �� |
��1����6.0gNa2Cr2O7����100mL�ձ��У���30mLˮ�ܽ⣬�ٻ�������5mLŨ���ᣬ��������ҺС��ת����B�У���A�м���4.0g�������ͼ�����ʯ�����ȣ�������������ʱ����ʼ�μ�B����Һ���μӹ����б��ַ�Ӧ�¶�Ϊ90-95�棬��E���ռ�90�����µ���֣�
��2��������ﵹ���Һ©���У���ȥˮ�㣬�л������������ռ�75-77����֣�����2.0g��
�ش��������⣺
��1��ʵ���У��ܷ�Na2Cr2O7��Һ�ӵ�Ũ�����в�˵�����ɲ��ܣ���ΪŨ������ܶȴ�������ˮ�ų��������ȣ��������Ž���
��2�������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ��������ȴ�ӣ�
��3������װ��ͼ�У�D������������ֱ�������ܣ�
��4����Һ©��ʹ��ǰ������еIJ�����c������ȷ�𰸱�ţ���
a����ʪb������ c����© d���궨
��5��������ȩ�ֲ�Ʒ���ڷ�Һ©���з�ˮʱ��ˮ���²㣨��ϡ����¡���
��6����Ӧ�¶�Ӧ������90-95�棬��ԭ���DZ�֤����ȩ��ʱ�������ֿɾ��������䱻��һ��������
��7����ʵ���У�����ȩ�IJ���Ϊ51%��
 �����״���C6H5��3C-OH��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壮ʵ���Һϳ������״���ʵ��װ����ͼ��ʾ��
�����״���C6H5��3C-OH��һ����Ҫ�Ļ���ԭ�Ϻ�ҽҩ�м��壮ʵ���Һϳ������״���ʵ��װ����ͼ��ʾ����֪��
�ٹ��������ɵ��м����ʸ����Լ�����ˮ�ⷴӦ��
�ڲ���������ʵķе����£�
| ���� | �е�/�� |
| �����״� | 380 |
| ���� | 34.6 |
| �屽 | 156.2 |
��ش��������⣺
��1��װ���в�������B������Ϊ�����ܣ�װ����ˮCaCl2������A�������Ƿ�ֹ�����е�ˮ��������װ�ã���������Լ�ˮ�⣮
��2��װ���еμ�Һ��δ����ͨ��Һ©�����õ�Һ©����������ƽ��ѹǿ��ʹ©����Һ��˳�����£���ȡ�����Լ�ʱҪ�����¶�ԼΪ40�棬���Բ���ˮԡ���ȷ�ʽ��
��3���Ƶõ������״��ֲ�Ʒ�к������ѡ��屽���Ȼ�淋����ʣ�������������ᴿ������
�ֲ�Ʒ$\stackrel{�ٲ���}{��}$$\stackrel{���ܽ⡢����}{��}$$\stackrel{��ϴ�ӡ�����}{��}$�����״������У������ٵ���������������ϴ��Һ���ѡ��a������ĸ��ţ���
a��ˮ b������ c���Ҵ� d����
�����Ʒ�Ѿ�ϴ�Ӹɾ��IJ���Ϊȡ�������һ��ϴ��Һ���Թ��У��μ���������Һ�����������ɣ�����ϴ�Ӹɾ�����֮��δϴ�Ӹɾ���
��4�����Ȳⶨ����ȡ2.60g��Ʒ�����������Һ���������������ƣ��������Ʋ���Ӧ������ַ�Ӧ������ɵ������ڱ�״���µ����Ϊ100.80mL�����Ʒ�������״�����������Ϊ90%��
| A�� | ������������Ȼ�л��߷��ӻ����û�е����ʾ�û������ | |
| B�� | HCHO��Һ��NH4��2SO4��Һ����ʹ�����ʱ��� | |
| C�� | ijЩ�����ʸ�Ũ�������û��� | |
| D�� | ���Բ��ö���������������ķ������롢�ᴿ������ |


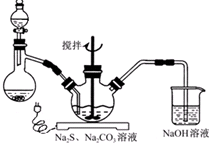 NaCNΪ�綾���ij��ѧ��ȤС��������ϵ�֪��ʵ��������軯����Һ��ʹ�������������Һ����ͳһ�ⶾ���٣����ǿ�չ����������ʵ�飬����Ҫ��ش����⣺
NaCNΪ�綾���ij��ѧ��ȤС��������ϵ�֪��ʵ��������軯����Һ��ʹ�������������Һ����ͳһ�ⶾ���٣����ǿ�չ����������ʵ�飬����Ҫ��ش����⣺