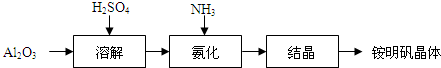
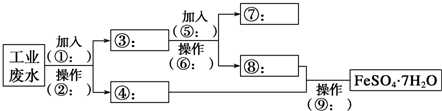
��Ŀ����
��27.4g Na2CO3��NaHCO3�Ļ����ƽ���ֳɵ��������ݣ�һ������ˮ���������ijŨ�ȵ����ᣬ�ռ���CO2����V L����������100mL����һ��ֱ�Ӽ��������أ�����CO2����1.12L����������������ڱ�״���²ⶨ�����Լ��㣺
��1��ԭ��Ϲ�����Na2CO3��NaHCO3�����ʵ���֮�ȣ�n��Na2CO3����n��NaHCO3��= ��
��2��V= L����������ʵ���Ũ��c��HCl��= mol?L-1��
��1��ԭ��Ϲ�����Na2CO3��NaHCO3�����ʵ���֮�ȣ�n��Na2CO3����n��NaHCO3��=
��2��V=
���㣺�йػ���ﷴӦ�ļ���
ר�⣺
��������1�������ֱ�Ӽ���ʱֻ��NaHCO3�ֽ⣬������Ӧ2NaHCO3
Na2CO3+CO2��+H2O�����ݷ���ʽ֪��n��NaHCO3��=2n��CO2��=2��
=0.1mol��m��NaHCO3��=0.1mol��84g/mol=8.4g����m��Na2CO3��=
-8.4g=5.3g��n��Na2CO3��=
=0.05mol��
��2��������ϡ���ᷴӦ����CO2������Cԭ���غ��n��Na2CO3��+n��NaHCO3��=n��CO2��=
mol���ݴ˼��������̼�������Ӧ����Һ�е�����ΪNaCl������Clԭ���غ����HCl�����ʵ���Ũ�ȣ�
| ||
| 1.12L |
| 22.4L/mol |
| 27.4g |
| 2 |
| 5.3g |
| 106g/mol |
��2��������ϡ���ᷴӦ����CO2������Cԭ���غ��n��Na2CO3��+n��NaHCO3��=n��CO2��=
| V |
| 22.4 |
���
�⣺��1�������ֱ�Ӽ���ʱֻ��NaHCO3�ֽ⣬������Ӧ2NaHCO3
Na2CO3+CO2��+H2O�����ݷ���ʽ֪��n��NaHCO3��=2n��CO2��=2��
=0.1mol��m��NaHCO3��=0.1mol��84g/mol=8.4g����m��Na2CO3��=
-8.4g=5.3g��n��Na2CO3��=
=0.05mol������n��Na2CO3����n��NaHCO3��=0.05mol��0.1mol=1��2���ʴ�Ϊ��1��2��
��2��������ϡ���ᷴӦ����CO2������Cԭ���غ��n��Na2CO3��+n��NaHCO3��=n��CO2��=
mol=0.05mol+0.1mol������V=0.15mol��22.4L/mol=3.36L��
��Ӧ����Һ�е�����ΪNaCl������Naԭ���غ��n��NaCl��=2n��Na2CO3��+n��NaHCO3��=0.1mol+0.1mol=0.2mol������Clԭ���غ��n��HCl��=n��NaCl��=0.2mol����C��HCl��=
=2.0mol/L���ʴ�Ϊ��3.36��2.0��
| ||
| 1.12L |
| 22.4L/mol |
| 27.4g |
| 2 |
| 5.3g |
| 106g/mol |
��2��������ϡ���ᷴӦ����CO2������Cԭ���غ��n��Na2CO3��+n��NaHCO3��=n��CO2��=
| V |
| 22.4 |
��Ӧ����Һ�е�����ΪNaCl������Naԭ���غ��n��NaCl��=2n��Na2CO3��+n��NaHCO3��=0.1mol+0.1mol=0.2mol������Clԭ���غ��n��HCl��=n��NaCl��=0.2mol����C��HCl��=
| 0.2mol |
| 0.1L |
���������⿼�����ﷴӦ���йؼ��㣬���ؿ����������������������ԭ���غ���м��㣬֪������֮��ķ�Ӧ����Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
ij�о�С��Ϊ�ⶨʳ�ð״��д���ĺ������е����²�������ȷ���ǣ�������
| A���ü�ʽ�ζ�����ȡһ������Ĵ���״�����ƿ�� |
| B����ȡ4.0gNaOH��1000mL����ƿ��ˮ���̶ȣ����1.00 mol?L-1NaOH����Һ |
| C����NaOH��Һ�ζ��״ף�ʹ�÷�̪��ָʾ������Һ��ɫǡ������ɫ��Ϊdz��ɫ���Ұ�����ڲ���ɫʱ��Ϊ�ζ��յ� |
| D���ζ�ʱ�۾�Ҫע���ŵζ�����NaOH��Һ��Һ��仯����ֹ�ζ����� |
������������Ϊm g������þ�Ļ����ֱ�Ͷ�뵽������NaOH��Һ�������У�����H2��ͬ��ͬѹ�µ������Ϊ1��2����ԭ�����������þ�����ʵ���֮��Ϊ��������
| A��1��2 | B��1��3 |
| C��3��1 | D��2��3 |
 ����ֻ�ܱ��������ң���������һ�������ƶ��Ļ�����ʹ�����ڱ��ֺ�ѹ���������ܱ��ֺ��ݣ���ʼʱ�������������зֱ��������ʵ����������Ϊ2��1��M��N�Ļ�����壬��ʹ�����ݻ���ȣ�����ͼ��ʾ�����ڱ���400���������ʹ֮�������·�Ӧ��2M��g��+N��g��?2Q��g�������ﵽƽ�⣮����˵����ȷ���ǣ�������
����ֻ�ܱ��������ң���������һ�������ƶ��Ļ�����ʹ�����ڱ��ֺ�ѹ���������ܱ��ֺ��ݣ���ʼʱ�������������зֱ��������ʵ����������Ϊ2��1��M��N�Ļ�����壬��ʹ�����ݻ���ȣ�����ͼ��ʾ�����ڱ���400���������ʹ֮�������·�Ӧ��2M��g��+N��g��?2Q��g�������ﵽƽ�⣮����˵����ȷ���ǣ�������| A���������ﵽƽ��ʱ�����ʱ����������� |
| B��ƽ��ʱ��������M��ת���ʱ�������С |
| C��ƽ���������������ͨ����������ĵ����ʵ�����������������л�ѧƽ�����淴Ӧ�����ƶ����������л�ѧƽ�ⲻ�ƶ� |
| D��ƽ���������������ͨ������ʵ�����ԭ��Ӧ���壬�ﵽƽ��ʱ���������Ļ��������Q�����������С���������Ļ��������Q������������� |
��֪��C��s��+O2��g��=CO2��g����H1
CO2��g��+C��s��=2CO��g����H2
2CO��g��+O2��g��=2CO2��g����H3
4Fe��s��+3O2��g��=2Fe2O3��s����H4
3CO��g��+Fe2O3��s��=3CO2��g��+2Fe��s����H5
���й���������Ӧ�ʱ���ж���ȷ���ǣ�������
CO2��g��+C��s��=2CO��g����H2
2CO��g��+O2��g��=2CO2��g����H3
4Fe��s��+3O2��g��=2Fe2O3��s����H4
3CO��g��+Fe2O3��s��=3CO2��g��+2Fe��s����H5
���й���������Ӧ�ʱ���ж���ȷ���ǣ�������
| A����H1��0����H2��0 |
| B����H3��0����H4��0 |
| C����H3=��H1-��H2 |
| D����H4=2��H5-3��H3 |