��Ŀ����
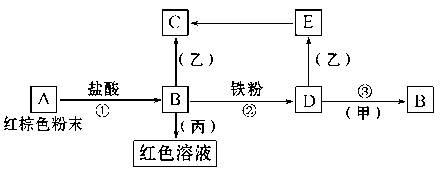
��֪A��K��Ϊ��ѧ��ѧ�еij������ʣ�����֮���ת����ϵ��ͼ��ʾ������A��DΪ�������ʣ���Ӧ���������ɵ�ˮ���������ֲ�������ȥ��
��ش��������⣺
��1��A��B��Ӧ�Ļ�ѧ����ʽ�ǣ�______________________________________��
��2����F��ͨ������CO2����K�����ӷ���ʽΪ��_________________________��
��3��A��NaOH��Һ��Ӧ�����ӷ���ʽ�ǣ�_________________________________________________��
��4���ټ�������J����Һ�н������ӵķ����ǣ�_______________________________________________��
�ڽ�����E����ˮ������Һ�����ԣ�ԭ���ǣ������ӷ���ʽ��ʾ��____________��
��ij��Ч��ˮ������D��OH��SO4�ۺϵõ��ġ���ҵ����DSO4��ϡ�������������Ϊԭ�����Ʊ�D��OH��SO4����Ӧ����NO���ɣ��÷�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��1��Fe2O3+2Al Al2O3+2Fe
Al2O3+2Fe
��2��
��3��
��4����ȡ������Һ���Թ��У��μӼ���KSCN��Һ����Һ���ɫ����֤��ԭ��Һ�к���Fe3+
��
��2FeSO4+2NaNO2+H2SO4=2Fe��OH��SO4+Na2SO4+2NO��
����

����X����������������ͭ��ɣ�ȡ������ȵ�������������ͼ����ʵ�飺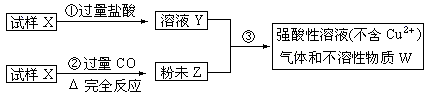
��1����д�����ۢ��з�����ȫ����Ӧ�����ӷ���ʽ��____________________________________��
��2��Ҫʹ����Xת��Ϊ��ĩZ������CO�⣬������ʹ�� ��
| A������ | B����̿ | C������ | D������ |