��Ŀ����
18�������л���A��B��C��D��E������A��B�����࣬����Ϊ���������A��E̼����ͬ��B��D��һ��̼ԭ�ӣ��һ�Ϊͬϵ����³�ѹ��AΪ���壬B��C��D��E��Һ�壮��֪����1��A���Ծۺϣ���ȫȼ��1molA��������3mol��
��2��B������������������ܶ���39����ȫȼ��7.8g B��������16.8L����״̬����
��3��C��Ũ���Ṳ�ȵ�A��C�������ɵ�E��
��4��E��Na2CO3��Һ��������������
���������ƶϻش����У�
�ٸ����ʵĽṹ��ʽ��
ACH2=CH2B
 CCH3CH2OHD
CCH3CH2OHD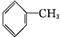 ECH3COOH
ECH3COOH��д�����л�ѧ����ʽ
����C��������2CH3CH2OH+O2$��_{��}^{Cu/Ag}$2CH3COOH
����B��Ũ���ᡢŨ�����ϼ��ȣ�

����C��E��Ũ�����ϼ��ȣ�CH3COOH+CH3CH2OH
 CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
���� ��A�ķ���ʽΪCxHy����ȫȼ��1molA��������3mol������x+$\frac{y}{4}$=3�����³�ѹ��AΪ���壬��x��4��A���Ծۺϣ�A��̼̼˫����̼̼�μ�������AΪC2H4���ṹ��ʽΪCH2=CH2��B������������������ܶ���39������B����ͨ�Է�������Ϊ78����ȫȼ��7.8g B��������16.8L����״̬����BΪҺ̬������B�ķ���ʽΪCaHb����x+y/4=$\frac{16.8}{\frac{22.4}{\frac{7.8}{78}}}$=7.5��a��4���ɵ�B�ķ���ʽΪC6H6���ṹ��ʽΪ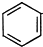 ��C��Ũ���Ṳ�ȵ���ϩ��C�������ɵ�E��E��Na2CO3��Һ������������������CΪCH3CH2OH��EΪCH3COOH��B��D�ֱ�̼����ͬ������6��̼ԭ�ӣ�DΪ���壬D��Һ��FeCl3��Һ����ɫ��˵���DZ��ӣ��ݴ˴��⣮
��C��Ũ���Ṳ�ȵ���ϩ��C�������ɵ�E��E��Na2CO3��Һ������������������CΪCH3CH2OH��EΪCH3COOH��B��D�ֱ�̼����ͬ������6��̼ԭ�ӣ�DΪ���壬D��Һ��FeCl3��Һ����ɫ��˵���DZ��ӣ��ݴ˴��⣮
��� �⣺�٣�1������ȼ�յ�ͨʽΪ��CxHy+��x+$\frac{y}{4}$��O2$\stackrel{��ȼ}{��}$xCO2+$\frac{y}{2}$H2O��A�����࣬��A�ķ���ʽΪCxHy����ȫȼ��1molA��������3mol������x+$\frac{y}{4}$=3�����³�ѹ��AΪ���壬��x��4��A���Ծۺϣ�A��̼̼˫����̼̼�μ�������AΪC2H4���ṹ��ʽΪCH2=CH2��
��2���ɦ�=$\frac{m}{v}$=$\frac{M}{{V}_{m}}$��֪������ͬ�������������Է�������֮�ȵ����ܶ�֮�ȣ��û����������������������ܶ�Ϊ39����û��������Է�������Ϊ39��2=78��7.8g B�����ʵ���Ϊn=$\frac{m}{M}$=$\frac{7.8g}{78g/mol}$=0.1mol��16.8L��������״̬�������ʵ���Ϊn=$\frac{V}{{V}_{m}}$=$\frac{16.8L}{22.4L}$=0.75mol����B�ķ���ʽΪCaHb��a+$\frac{b}{4}$=7.5��BΪҺ̬����a��4��a=6��b=6��B�ķ���ʽΪC6H6���ṹ��ʽΪ ��
��
��3��C��Ũ���Ṳ�ȵ���ϩ��C�������ɵ�E��E��Na2CO3��Һ������������������CΪCH3CH2OH���Ҵ���Ũ���������¼��ȷ�����ȥ��Ӧ����ϩ����Ӧ����ʽΪ��CH3-CH2-OH$��_{170��}^{Ũ����}$CH2=CH2��+H2O��C�������ɵ�E��2CH3CH2OH+O2$��_{��}^{Cu/Ag}$2CH3COOH��
��4��������̼���Ʒ�Ӧ���������ơ�������̼��ˮ����ѧ����ʽΪ2CH3COOH+Na2CO3=2CH3COONa+CO2��+H2O��
�ʴ�Ϊ��CH2=CH2�� �� CH3CH2OH��
�� CH3CH2OH�� ��CH3COOH��
��CH3COOH��
�ڢ���CΪCH3CH2OH��C�������ɵ�E����Ӧ����ʽΪ��2CH3CH2OH+O2$��_{��}^{Cu/Ag}$2CH3COOH��
�ʴ�Ϊ��2CH3CH2OH+O2$��_{��}^{Cu/Ag}$2CH3COOH��
��������Ũ������Ũ������������50�桫60����������·���ȡ����Ӧ��������������Ӧ����ʽΪ ��
��
�ʴ�Ϊ�� ��
��
����CΪCH3CH2OH��EΪCH3COOH����Ũ���������������¼����Ҵ������ᷢ��������Ӧ�������ǻ��������⣬����������������Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
�ʴ�Ϊ��CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O��
CH3COOCH2CH3+H2O��
���� ������Ҫ�����л�����ƶϣ���Ϥ�������ʵĽṹ�������Լ����ݹ����ŵ����ʺ�ȼ�պ������������ʽṹ��ȷ���ǽ��ؼ����ܽϺõĿ���ѧ���ķ�����˼ά��������Ŀ�Ѷ��еȣ�

 ������Ȳ��������Ϊ��������
������Ȳ��������Ϊ��������| A�� | 5-��-3-�һ�-1-��Ȳ | B�� | 5-��-3-�һ�-2-��Ȳ | ||
| C�� | 4-��-5-�һ�-2-��Ȳ | D�� | 2-��-4-�һ�-5-��Ȳ |
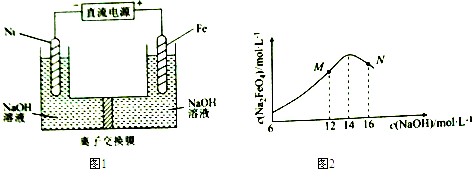
��֪��FeO42-Ϊ�Ϻ�ɫ��Na2FeO4ֻ��ǿ�����������ȶ����ױ�H2��ԭ����Һ��OH-Ũ�ȹ��ߣ����缫����������ɫ���ʣ�����˵��������ǣ�������
| A�� | �Ʊ�Na2FeO4�ĵ缫��ӦΪFe-6e-+8OH-=FeO42-+4H2O | |
| B�� | �������У��뽫�������������弰ʱ�ų� | |
| C�� | MN������c��Na2FeO4���������ֵ��ԭ��ͬ����M�����Fe��OH��3���� | |
| D�� | ͼ1�е����ӽ���ĤΪ�����ӽ���Ĥ���������У�����������pH������ |
| A�� | ������ | B�� | ������ | C�� | ���ʻ� | D�� | ��ά�� |
| A�� | ������ɫ������һ�����ɫ������ɫ��˵����Һ����HC1O���� | |
| B�� | ��Һ�ʻ���ɫ�����д̼�����ζ��˵����C12���Ӵ��� | |
| C�� | ���������ữ��AgNO3��Һ������ɫ������˵����C1-���� | |
| D�� | ����NaOH��Һ����ˮ����ɫ��ʧ��˵����HC1O���Ӵ��� |
����NaI��Һ��ͨ������ʵ��ٲ��������壬��Һ���ɫ��
��ȡʵ������ɵ���Һ���ڵ���KI��ֽ�ϣ���ֽ����ɫ��
�����жϲ���ȷ���ǣ�������
| A�� | ʵ���˵��KI������ | |
| B�� | ʵ������������뻹ԭ�������ʵ���֮��Ϊ1��2 | |
| C�� | ʵ���֤��Cl-���л�ԭ�� | |
| D�� | ����ʵ֤�������ԣ�ClO3-��Cl2��I2 |
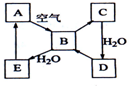 ��ͼ��ʾij��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ����������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϣ�
��ͼ��ʾij��̬����A���仯����֮���ת����ϵ��ijЩ����ͷ�Ӧ��������ȥ����������B�ڳ��³�ѹ��Ϊ���壬B��C����Է�������֮��Ϊ4��5��������D����Ҫ�Ĺ�ҵԭ�ϣ� ��
��