��Ŀ����
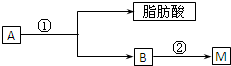
�Ų��������Ļ�ѧ��������������Fe2O3�������ǵ��ӡ����Ź�ҵ�Ĵ��Բ��ϣ���ҵ�ϲ��������Ѱ۵��½��ϣ�������FeSO4�ķ�Һ��Ϊԭ�����Ʊ��Ų���������

��֪�����еĻ�ѧ��Ӧ����ʽΪ��FeCO3��FeO+CO2����4FeO+O2��2Fe2O3
��1����98%��H2SO4������500mL��20%��H2SO4�����貣�������� ��
A�������� B���ձ� C��©�� D��250mL����ƿ E��500mL����ƿ F����ͷ�ι�
��2��Ũ���ᾧ��õ��ľ����� ���ѧʽ����A�������� ������Һ�и����ӵ�Ũ�ȴ�СΪ�� ��
��3��20%H2SO4����Ƥ�����÷ֱ��� ��
��4��������Һ�к���NH4+�ķ����� ��
��5��д�����衰�ϳɡ��з����Ļ�ѧ�仯���û�ѧ����ʽ��ʾ���� ��

��֪�����еĻ�ѧ��Ӧ����ʽΪ��FeCO3��FeO+CO2����4FeO+O2��2Fe2O3
��1����98%��H2SO4������500mL��20%��H2SO4�����貣��������
A�������� B���ձ� C��©�� D��250mL����ƿ E��500mL����ƿ F����ͷ�ι�
��2��Ũ���ᾧ��õ��ľ�����
��3��20%H2SO4����Ƥ�����÷ֱ���
��4��������Һ�к���NH4+�ķ�����
��5��д�����衰�ϳɡ��з����Ļ�ѧ�仯���û�ѧ����ʽ��ʾ����
���㣺���ʷ�����ᴿ�ķ����ͻ��������ۺ�Ӧ��,�������������������
ר�⣺������Ҫ�Ľ������仯����
�������½����к���������������ϡ���ᡢ��Ƭ������Ӧ����������������ϡ������������������ˮ�⣬Ȼ������Һ�м�����������ˮ���õ��������ʵ���������Ȼ����˵õ���Һ��Ũ���ᾧ��Һ���õ�FeSO4?7H2O��Ȼ������Һ�м���̼����泥������ķ�ӦΪFeSO4+2NH4HCO3=FeCO3��+�� NH4��2 SO4+CO2��+H2O��Ȼ��ϴ�ӹ��˵õ�A����ҺAΪ��NH4��2 SO4��������FeCO3���յõ�Fe2O3���ٽ����Ŀ�������
���
�⣺�½����к���������������ϡ���ᡢ��Ƭ������Ӧ����������������ϡ������������������ˮ�⣬Ȼ������Һ�м�����������ˮ���õ��������ʵ���������Ȼ����˵õ���Һ��Ũ���ᾧ��Һ���õ�FeSO4?7H2O��Ȼ������Һ�м���̼����泥������ķ�ӦΪFeSO4+2NH4HCO3=FeCO3��+�� NH4��2 SO4+CO2��+H2O��Ȼ��ϴ�ӹ��˵õ�A����ҺAΪ��NH4��2 SO4��������FeCO3���յõ�Fe2O3��
��1����98%��H2SO4������500mL��20%��H2SO4�����貣���������������ͽ������õIJ�������ʢ����Һ���ձ���500mL����ƿ���������õĽ�ͷ�ιܣ���ѡABEF��
��2��ͨ�����Ϸ���֪��Ũ���ᾧ��õ��ľ�����FeSO4?7H2O��A�������� �� NH4��2 SO4���������ǿ�������Σ�笠�����ˮ�����Һ�����ԣ���ˮ��̶Ƚ�С�����ݵ���غ��c��NH4+����c��SO42-��������Һ�и����ӵ�Ũ�ȱȽϴ�СΪ��c��NH4+����c��SO42-����c��H+����c��OH-����
�ʴ�Ϊ��FeSO4?7H2O�� �� NH4��2 SO4��c��NH4+����c��SO42-����c��H+����c��OH-����
��3���������ӱ�������������Ƭ��ֹ�������ӱ�����������������ˮ�⣬Ϊ��ֹˮ�����ϡ���ᣬ����20%H2SO4����Ƥ�����÷ֱ�����������Fe2+���Ƶ�ˮ�⣬��Ƥ�����÷�ֹFe2+���������ʴ�Ϊ����������Fe2+���Ƶ�ˮ�⣬��Ƥ�����÷�ֹFe2+��������
��4��������Һ�к���NH4+�ķ������Թ���ȡ��Һ����������������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬����ֽ����ɫ����֤����Һ�к���NH4+���ʴ�Ϊ�����Թ���ȡ��Һ����������������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬����ֽ����ɫ����֤����Һ�к���NH4+��
��5��ͨ�����Ϸ���֪���÷�Ӧ����ʽΪFeSO4+2NH4HCO3=FeCO3��+�� NH4��2 SO4+CO2��+H2O���ʴ�Ϊ��FeSO4+2NH4HCO3=FeCO3��+�� NH4��2 SO4+CO2��+H2O��
��1����98%��H2SO4������500mL��20%��H2SO4�����貣���������������ͽ������õIJ�������ʢ����Һ���ձ���500mL����ƿ���������õĽ�ͷ�ιܣ���ѡABEF��
��2��ͨ�����Ϸ���֪��Ũ���ᾧ��õ��ľ�����FeSO4?7H2O��A�������� �� NH4��2 SO4���������ǿ�������Σ�笠�����ˮ�����Һ�����ԣ���ˮ��̶Ƚ�С�����ݵ���غ��c��NH4+����c��SO42-��������Һ�и����ӵ�Ũ�ȱȽϴ�СΪ��c��NH4+����c��SO42-����c��H+����c��OH-����
�ʴ�Ϊ��FeSO4?7H2O�� �� NH4��2 SO4��c��NH4+����c��SO42-����c��H+����c��OH-����
��3���������ӱ�������������Ƭ��ֹ�������ӱ�����������������ˮ�⣬Ϊ��ֹˮ�����ϡ���ᣬ����20%H2SO4����Ƥ�����÷ֱ�����������Fe2+���Ƶ�ˮ�⣬��Ƥ�����÷�ֹFe2+���������ʴ�Ϊ����������Fe2+���Ƶ�ˮ�⣬��Ƥ�����÷�ֹFe2+��������
��4��������Һ�к���NH4+�ķ������Թ���ȡ��Һ����������������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬����ֽ����ɫ����֤����Һ�к���NH4+���ʴ�Ϊ�����Թ���ȡ��Һ����������������NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ���飬����ֽ����ɫ����֤����Һ�к���NH4+��
��5��ͨ�����Ϸ���֪���÷�Ӧ����ʽΪFeSO4+2NH4HCO3=FeCO3��+�� NH4��2 SO4+CO2��+H2O���ʴ�Ϊ��FeSO4+2NH4HCO3=FeCO3��+�� NH4��2 SO4+CO2��+H2O��
������������������Ϊ���忼������ķ�����ᴿ�����ؿ������������������漰����ˮ�⡢������ԭ��Ӧ��ʵ�������֪ʶ�㣬��ȷʵ��ԭ���ǽⱾ��ؼ���֪�����Ӽ��鷽��������ͼ��ÿһ�������ķ�Ӧ����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 Ӧ�����������Ĵ���ѧ������ϵ�д�
Ӧ�����������Ĵ���ѧ������ϵ�д�
�����Ŀ
ʵ���ú������ʣ�FeO��Fe2O3���ķ�CuO�Ʊ��������壬�������й��̣���֪Fe3+��pH=5ʱ����ȫ���������з���������ǣ�������
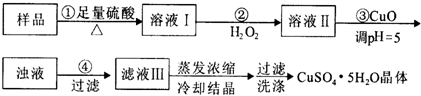

| A������CuCO3���CuOҲ�ɵ�����Һ��pH����Ӱ��ʵ���� |
| B��������з�������Ҫ��ӦΪ��H2O2+2Fe2++2H+=2Fe3++2H2O |
| C��ϴ�ӣ���װ�����©���м�ˮ����û���壬����Ȼ���º��ظ�2��3�� |
| D��ijʵ����Ҫ240 mL1mol/L��CuSO4��Һ��������ʱ�����CuSO4?5H2O 60g |
���������Ӹֹ������ã�������
| A����ͬ������Ӧ�����ȶ��ı����� |
| B�������ȵ������� |
| C�����Ͻ�ǿ�ȴ� |
| D������ǿ��ԭ�������ȷ�Ӧ���� |
������Ԫ��X��Y��Z��W��ԭ����������������ԭ������������֮��Ϊ13��X��ԭ�Ӱ뾶��Y��С��X��Wͬ���壬Zԭ�ӵ����������������ڲ��������3��������˵����ȷ���ǣ�������
| A��Ԫ��Z��W�ļ����ӵĵ��Ӳ�ṹ��ͬ |
| B��Ԫ�صķǽ�������ǿ������Y��Z��X |
| C��ֻ��X��Y��Z ����Ԫ�صĻ�������������ӻ����Ҳ�����ǹ��ۻ����� |
| D��Ԫ��Y����̬�⻯����������������Ӧ��ˮ���ﲻ��Ӧ |