��Ŀ����
6��ij����С�����Fe��OH��3������Ʊ�ʵ�鲢���������ʣ���1�����мס��ҡ�������ͬѧ����Fe��OH��3������Ʊ���
�ټ�ͬѧ�IJ����ǣ�ȡһС�ձ�������25mL����ˮ���������ڣ����ˮ����μ���1��2mL FeCl3������Һ��������������Һ�ʺ��ɫ��ֹͣ���ȣ�
����ֱ�Ӽ��ȱ���FeCl3��Һ��
�۱����ˮ�еμӱ���FeCl3��Һ��Ϊ��ʹ��Ӧ���г�֣����10���ӣ�
����Ϊ��λͬѧ���Ʊ���������������ͬѧ���������ý����ʲô������֤����Fe��OH��3�������ɣ������ЧӦ��
��2��Fe��OH��3�����ȶ����ڵ���Ҫԭ����B��
A������ֱ��С��1nm B�������������C�������������˶� D������������ֽ
��3��Fe��OH��3����������FeCl3��Һ��ʵ�������A��
A��Fe��OH��3�������ӵ�ֱ����1��100nm֮�� B��Fe��OH��3������ж����ЧӦ
C��Fe��OH��3�����Ǿ�һ�ķ�ɢϵ D��Fe��OH��3����ķ�ɢ��������ֽ
��4���ᴿ����Fe��OH��3���峣�õķ�����������������Fe��OH��3�����е�������ֱ�������������˹��̵�ʵ��������������ʱ���к��ɫ�������ɣ��������ʱ�������ܽ⣬��Һ����ػ�ɫ��
���� ��1��ʵ�����Ʊ����������������ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱӦ����ֹͣ���ȣ���������Ȼᵼ�½���۳���������ж�������ʣ�������������ɢϵ�Ķ������ʣ�
��2������������ɣ���ų⣬�����ײ����ϴ����Ӷ��۳���
��3������������������ɢϵ�ı��������Ƿ�ɢ����ֱ���Ĵ�С��ͬ��
��4�����岻������Ĥ��������ֽ����Һ����ͨ����Ĥ�����������ķ������뽺�����Һ����Fe��OH��3�����е���ϡ���ᣬ�ȷ�������ľ۳���Ȼ�����֮��ᷢ�����ֽⷴӦ��
��� �⣺��1����ʵ�����Ʊ����������������ڷ��ڵ�����ˮ�м��뱥���Ȼ�����Һ������Һ��Ϊ���ɫʱ����ֹͣ���ȣ�����Һ��Ϊ���ɫʱӦ����ֹͣ���ȣ���������Ȼᵼ�½���۳����ʼ���ȷ��
����ֱ�Ӽ��ȱ���FeCl3��Һ��ٽ��Ȼ���ˮ�⣬�Ҽ��ȴٽ�HCl�ӷ���������Һ�������������Ҵ���
�ۼ������10���ӻ�����������ʱ�����
������ж����ЧӦ�����ü��������ʱ������һ�������Ĺ�·��������������ɢϵ�Ķ������ʣ�����һ������ͨ���Ƶõ�Fe��OH��3���壬�Ӳ���۲쵽һ�������ġ�ͨ·����˵���Ѿ��Ƶý��壬
�ʴ�Ϊ����ͬѧ�������ЧӦ��
��2��������к�ǿ��������������������Һ�е����Ӷ�����ɣ�����֮����ų⣬�����ײ����ϴ������۳���
�ʴ�Ϊ��B��
��3��Fe��OH��3����������FeCl3��Һ��ʵ�������Fe��OH��3����ķ�ɢ����ֱ����С��1��100nm֮�䣬
�ʴ�Ϊ��A��
��4����Һ����ͨ����Ĥ�����岻������Ĥ���ᴿFe��OH��3����ķ�������������Fe��OH��3�����е���ϡ���ᣬ����ᷢ���۳����������ʱ��Fe��OH��3�����ᷢ�����ֽⷴӦ����������ʧ��
�ʴ�Ϊ������������������ʱ���к��ɫ�������ɣ��������ʱ�������ܽ⣬��Һ����ػ�ɫ��
���� ���⿼�齺����й�֪ʶ��Ϊ�߿��������ͣ�������ѧ���ķ���������ʵ�������Ŀ��飬��Ŀ�ѶȲ���ע����ս�����Ʊ��������Լ���Һ�ͽ���ļ����ᴿ�ȸ�Ƶ���㣮

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ˮ��Ħ��������18g | B�� | 1���������������98g | ||
| C�� | 1molN2��������28g | D�� | Ħ�����߸�����������֮һ |
| A�� | ��״����44.8L�Ҵ���C2H5OH��������ԭ����Ϊ12NA | |
| B�� | 25��C��101KPa�����£�1.4g N2���������1.12L | |
| C�� | 22gij���庬������Ϊ0.5NA������Ħ������Ϊ44 | |
| D�� | ��״����1.12L CO�� N2�Ļ�����庬��Լ3.01��1022��ԭ�� |
| A�� | OH-+AlO2-+2H+=Al��OH��3 | B�� | OH-+AlO2-+5H+=Al3++3H2O | ||
| C�� | 2OH-+AlO2-+3H+=Al��OH��3+H2O | D�� | OH-+2AlO2-+3H++H2O=2Al��OH��3 |
| A�� | ��Ȳ | B�� | ����ȩ | ||
| C�� | �����ϩ | D�� | ��ϩȩ��CH2=CH-CHO�� |
| A�� | �������ֱ��Ϊ10-9m��10-7m���� | |
| B�� | ʵ�����Ʊ�Fe��OH��3����ķ����ǽ�����FeCl3��Һ����NaOH��Һ������ | |
| C�� | ���Fe��OH��3���壬�����������ɫ�������Ϊ��������磬�������ƶ� | |
| D�� | �����ö����ЧӦ�����������Һ |
| A�� | 5 | B�� | 5.5 | C�� | 9 | D�� | 11 |
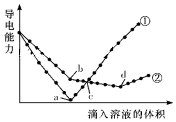 ��������ͬ��Ba��OH��2��Һ�У��ֱ�������ʵ���Ũ����ȵ�H2SO4��NaHSO4��Һ���䵼�������������Һ����仯��������ͼ��ʾ�����з�����ȷ���ǣ�������
��������ͬ��Ba��OH��2��Һ�У��ֱ�������ʵ���Ũ����ȵ�H2SO4��NaHSO4��Һ���䵼�������������Һ����仯��������ͼ��ʾ�����з�����ȷ���ǣ�������| A�� | �ڴ����μ�H2SO4��Һ�ı仯���� | |
| B�� | b����Һ�д������ڵ�������SO42-��Na+��OH- | |
| C�� | a��d�����Ӧ����Һ�������� | |
| D�� | c������Һ�к�����ͬ����OH- |
| A�� | K+��Fe3+��Cl- | B�� | Na+��CO32-��H+ | C�� | Na+��HCO3-��OH- | D�� | K+��AlO2-��OH- |