��Ŀ����
7�� ij�о���ѧϰС��Ϊ��֤����ͬ��ͬѹ�£���ͬŨ����ͬ��������Բ�ͬ��һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ��ʾ����ʵ�����Ҫ�����������£�
ij�о���ѧϰС��Ϊ��֤����ͬ��ͬѹ�£���ͬŨ����ͬ��������Բ�ͬ��һԪ��������þ����Ӧʱ�����������������ͬ����Ӧ���ʲ�ͬ��ͬʱ�ⶨʵ���������µ�����Ħ���������Ƶļ���ʵ��װ����ͼ��ʾ����ʵ�����Ҫ�����������£�������Ũ�Ⱦ�Ϊl mol•L-1������ʹ�����Һ��
������ʽ�ζ�����ȡ10.00mL l mol•L-1������ʹ�����Һ�ֱ���뵽������ƿ�У�
�۷ֱ��ȡ��ȥ��������Ĥ��þ��a g����ϵ��ͭ˿��ĩ�ˣ�a����ֵ����Ϊ0.12��
���ڹ��ƿ��װ������ˮ����ͼ��ʾ���Ӻ�װ�ã����װ�õ������ԣ�
�ݽ�ͭ˿�����ƶ���ʹ����þ���������У�ͭ˿������Ӵ���������Ӧ��ȫ����¼��Ӧ��ֹʱ�䣻
��Ӧ��������¶Ȼָ������£������������Һ����ƽ��������Ͳ��ˮ�����ΪV mL���뽫�������貹���������ش��������⣺
��1����ʵ����Ӧѡ��B������ţ�����Ͳ��
A��100mL B��200mL C��500mL
��2����ˮ������Ӱ����Բ��ƣ���ʵ���������£�����Ħ������ļ���ʽΪ��Vm=0.2VL/mol��
��3���������ʲ��ȵ�ԭ������������Ũ����ͬʱc��H+����ͬ��
���� �ڸ�����ʽ�ζ��ܺͼ�ʽ�ζ��ܵ�ʹ��Ҫ����ѡ��
�۸��ݻ�ѧ����ʽ���м��㣻
�ݱȽϻ�ѧ��Ӧ���ʣ������DZȽ�һ��ʱ��֮�ڵķ�Ӧ��Ũ�ȵı仯���������ı仯��
��1�����ݷ�Ӧ�Ļ�ѧ����ʽ�������ɱ���������������Ȼ���ж���Ͳ���
��2����������Ħ�����Vm=$\frac{V}{n}$���м��㣻
��3����Ӱ�컯ѧ��Ӧ���ʵ�����������������þ��ͭ���γ�ԭ��أ��ӿ췴Ӧ���ʣ�����ʵ��ⶨ��
��� �⣺������Ҫ��������10.00 mL�Ͼ�ȷ��Ӧѡ��ʽ�ζ��ܣ����ü�ʽ�ζ��ܻḯʴ�ܣ�
�ʴ�Ϊ����ʽ�ζ��ܣ�
��Mg+2HCl����HAc���TMgCl2+H2��
24g�� 2 mol����������������
a 1mol/L��0.01L����
a=0.12 g��
�ʴ�Ϊ��0.12��
�ݱȽϻ�ѧ��Ӧ���ʣ������DZȽ�һ��ʱ��֮�ڵķ�Ӧ��Ũ�ȵı仯���������ı仯��
�ʴ�Ϊ����Ӧ����ʱ�䣻
��1��Mg+2HCl����HAc���TMgCl2+H2��
�� 2 mol������������������1 mol
�� 1 mol/L��0.01 L���� n��H2��
n��H2��=0.005 mol��V��H2��=0.005 mol��22.4L/mol=0.112L=112ml��Ӧѡ200 mL��Ͳ��
�ʴ�Ϊ��B��
��2�������������ӵ����ʵ���Ϊ��1mol/L��0.01L���������������ʵ���Ϊ��1/2��1mol/L��0.01L��ͨ������������ΪVmL=V��10-3L��Vm=$\frac{V}{n}$=$\frac{V��1{0}^{-3}}{1/2��1��0.01}$=0.2VL/mol��
�ʴ�Ϊ��0.2VL/mol��
��3��Ӱ�컯ѧ��Ӧ���ʵ�������Ũ�ȡ��¶ȡ�ѹǿ�ȣ�����ΪH+Ũ�Ȳ�ͬ��þ��ͭ���γ�ԭ��أ��ӿ췴Ӧ���ʣ�����ʵ��ⶨ������ͭ˿������Ӵ���
�ʴ�Ϊ������������Ũ����ͬʱc��H+����ͬ��
���� ���⿼������������ʵ�鷽������ơ�̽��Ӱ�췴Ӧ���ʵ����ص�֪ʶ����Ŀ�Ѷ��еȣ�ע�����ջ�ѧʵ���������������Ӱ�컯ѧ��Ӧ���ʵ����أ���ȷ��������ʵ�鷽������Ʒ���������������ѧ���ķ���������������

��������������м��㣺
��1��ij�ȼ���ij����۵�����ÿСʱ��������485.92m3�����㵽��״������ͬ������2.169��104mol����֪����Cl2���������Ϊ0.985������ΪO2�������������ܶ�Ϊ3.144g/L����������NaOH4.273��104mol��������λ��Ч���֣���ͬ����
��2���±��ṩ��������ҺŨ�ȵı仯���ݣ������������������ϲ���ͨ������������Һ������仯����
| ������NaOH��Һ�������� | ������NaCl��ҺŨ�ȣ�g/L�� | |
| ����Һ | 0.30 | 310 |
| ���� | 0.32 | 210 |
��3����Ʒ֮һ----Ưˮ��NaClO��Һ���������õ�������Ư������������Ưˮ������ȡ1L��Һ�����pH=12������ˮ�⣩��NaClO����Ϊ0.3725g����һ�������£�������Һ�Ƴɾ��壬�������Ϊ1.335g��ͨ����ʽ���㣬д���þ���Ļ�ѧʽ��
| A�� |  ���ż� | B�� |  �в� | C�� |  ���� | D�� |  ������ |
��ʵ��ԭ����2KMnO4+5H2C2O4+3H2SO4�TK2SO4+2MnSO4+10CO2��+8H2O
��ʵ�����ݼ���¼��
| ʵ���� | �����£��Թ��������Լ���������/mL | ��Һ������ɫ����ʱ��/min | |||
| 0.6mol/L H2C2O4��Һ | H2O | 3mol/L ϡH2SO4��Һ | 0.05mol/L KMnO4��Һ | ||
| 1 | 3.0 | 2.0 | 2.0 | 3.0 | 1.5 |
| 2 | 2.0 | 3.0 | 2.0 | 3.0 | 2.7 |
| 3 | 1.0 | 4.0 | 2.0 | 3.0 | 3.9 |
��1�����ݱ��е�ʵ�����ݣ����Եõ��Ľ�����������������ʱ������С����Ӧ��Ũ�ȣ��ӿ죨��������ѧ��Ӧ���ʣ�
��2������ʵ��1�е����ݣ�������KMnO4��ʾ�Ļ�ѧ��Ӧ����Ϊ1.0��10-2mol/��L•min����
��3����С��ͬѧ���ݾ��������n��Mn2+����ʱ��仯��������ͼ1��ʾ������ͬѧ�������е�ʵ�����Ϸ��֣���ʵ�������n��Mn2+����ʱ��仯��ʵ��������ͼ2��ʾ��

��С��ͬѧ����ͼ2��ʾ��Ϣ������µļ��裬����������ʵ��̽����
�ٸ�С��ͬѧ����ļ�����Mn2+�Ը÷�Ӧ�д����ã�
�����������С��ͬѧ���ʵ�鷽��������д���пհ�
| ʵ���� | �����£��Թ��������Լ��������� | �����Թ��м���ij�ֹ��� | ��Һ������ɫ����ʱ��/min | |||
| 0.6mol/L H2C2O4��Һ | H2O | 3mol/L ϡH2SO4��Һ | 0.05mol/L KMnO4��Һ | |||
| 4 | 3.0 | 2.0 | 2.0 | 3.0 | MnSO4 | t |
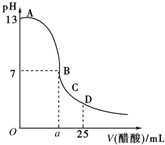 25��ʱ����25mL 0.1mol/L��NaOH��Һ�У���μ���0.2mol/L��CH3COOH��Һ����Һ��pH�仯����ͼ��ʾ�����з����Ľ�������ȷ���ǣ�������
25��ʱ����25mL 0.1mol/L��NaOH��Һ�У���μ���0.2mol/L��CH3COOH��Һ����Һ��pH�仯����ͼ��ʾ�����з����Ľ�������ȷ���ǣ�������| A�� | B��ĺ�����a=12.5 | |
| B�� | C��ʱ��Һ���У�c��Na+����c��CH3COO-����c��H+����c��OH-�� | |
| C�� | D��ʱ��Һ���У�c��CH3COO-��+c��CH3COOH��=2c��Na+�� | |
| D�� | ������A��B�������һ�㣬��Һ�ж��У�c��Na+����c��CH3COO-����c��OH-����c��H+�� |
 һ�ֻ�ѧ��Ϊ 2��4��4-����-2 �ǻ�-�����ѵ����ʣ���ͼ�������㷺Ӧ���ڷ�������������û�ѧƷ֮�У���ɱ�����������ã��������й�˵������ȷ���ǣ�������
һ�ֻ�ѧ��Ϊ 2��4��4-����-2 �ǻ�-�����ѵ����ʣ���ͼ�������㷺Ӧ���ڷ�������������û�ѧƷ֮�У���ɱ�����������ã��������й�˵������ȷ���ǣ�������| A�� | �����ʱ����ϵ�һ��ȡ������ 6 �� | |
| B�� | �������� FeCl3��Һ��Ϻ���ɫ | |
| C�� | ������������ԭ��һ������ͬһƽ�� | |
| D�� | ���ʵķ���ʽΪ C12H6Cl3O2 |

 ��֪1-�����ķе�Ϊ117.7�棬����ķе�Ϊ118�棬��ͬѧ����Ũ���������£�ʹ1-�������������������Ӧ�����ᶡ������Ӧ�¶�115��125�棩
��֪1-�����ķе�Ϊ117.7�棬����ķе�Ϊ118�棬��ͬѧ����Ũ���������£�ʹ1-�������������������Ӧ�����ᶡ������Ӧ�¶�115��125�棩