��Ŀ����
����ֲ���纣���������к��зḻ�ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڣ�ʵ������Ӻ�������ȡ���ʵ⣨I2����������ͼ�������Тܷ����Ļ�ѧ��ӦΪCl2+2KI�T2KCl+I2��
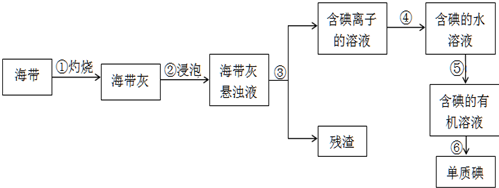
��1��ָ����ȡ�������ʵ������ۺ͢ݵ����ƣ� �� ��
��2���������ʹ�õIJ������������� ��ѡ����л��Լ������� ��
A���ƾ� B�����Ȼ�̼ C������ D����
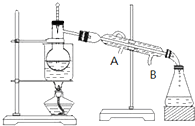
��3������Ӻ�����л���Һ����ȡ�Ⲣ�����л��ܼ�������Ҫ����������ͼ��ʾ������ˮ������Ϊ �� ������A��B��

��1��ָ����ȡ�������ʵ������ۺ͢ݵ����ƣ�
��2���������ʹ�õIJ�������������
A���ƾ� B�����Ȼ�̼ C������ D����
��3������Ӻ�����л���Һ����ȡ�Ⲣ�����л��ܼ�������Ҫ����������ͼ��ʾ������ˮ������Ϊ

���㣺��ˮ��Դ�����ۺ�����
ר�⣺ʵ����,Ԫ�ؼ��仯����
�������������տ�����ˮ�Ͷ�����̼���������к��е⻯�ص����ʣ�����ˮ�����˵õ����е����ӵ���Һ��������ˮ��ͨ�������������û����⣬�õ����ˮ��Һ���ñ������Ȼ�̼��ȡ��������ɵõ��⣬�Դ˽����⣮
��1�����ǹ����Һ������ù��˷���������ˮ��Һ�еⵥ�ʱ����Ȼ�̼��ȡ��ʵ�鷽����
��2�����ˮ��Һ���ñ������Ȼ�̼��ȡ��������ɵõ��⣬��Һ��ȡ��ʵ������Ҫ�÷�Һ©�����ձ���
��3����������IJ�����ˮͨ��Ϊ������
��1�����ǹ����Һ������ù��˷���������ˮ��Һ�еⵥ�ʱ����Ȼ�̼��ȡ��ʵ�鷽����
��2�����ˮ��Һ���ñ������Ȼ�̼��ȡ��������ɵõ��⣬��Һ��ȡ��ʵ������Ҫ�÷�Һ©�����ձ���
��3����������IJ�����ˮͨ��Ϊ������
���
�⣺��1�����ǹ����Һ������ù��˷���������ˮ��Һ�еⵥ�ʱ����Ȼ�̼��ȡ��ʵ�鷽�����ʴ�Ϊ�����ˣ���ȡ��Һ��
��2����Ϊ��ȡ�������õ���Һ©�����ձ���ѡ����л��Լ�Ӧ������ˮ��ֻ�б������Ȼ�̼���ϣ��ʴ�Ϊ����Һ©�����ձ���BD��
��3������ʱ��Ϊ���������Ӧ��B��ˮ��A��ˮ����ˮ��ˮ���������ܣ��õ����������Ŀ�ģ��ʴ�Ϊ��B��A��
��2����Ϊ��ȡ�������õ���Һ©�����ձ���ѡ����л��Լ�Ӧ������ˮ��ֻ�б������Ȼ�̼���ϣ��ʴ�Ϊ����Һ©�����ձ���BD��
��3������ʱ��Ϊ���������Ӧ��B��ˮ��A��ˮ����ˮ��ˮ���������ܣ��õ����������Ŀ�ģ��ʴ�Ϊ��B��A��
�����������ۺϿ������ʵķ�����ᴿ��������ѧ���ķ���������ʵ�������Ŀ��飬Ϊ�߿��������ͺ�Ƶ���㣬ע��Ӱ���ʵ�����̺�ʵ��ԭ�������ղ���������ע������ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
 ���ڹ�����ռ����Ҫ��λ��
���ڹ�����ռ����Ҫ��λ��
 ��Ӿ��ˮ�ĺ�������Ӧ�ÿ�����0.5mg/L��1.0mg/L֮�䣬
��Ӿ��ˮ�ĺ�������Ӧ�ÿ�����0.5mg/L��1.0mg/L֮�䣬
