��Ŀ����
�����к��Ȳⶨ��ʵ��ش��������⣺
��1�����ν�������ý����Ƶ�ԭ���� ��
��2����ͬһ�¶ȼƲ���������¶Ⱥ��ٲ�����������Һ�¶�ǰ����еIJ����ǣ� ��
��3��װ���д�С�ձ�����������ĭ���ϣ�����ֽ���������� ��
��4����֪ˮ�ı�����Ϊ4.18J/��g?�棩��ϡ�����ϡ����������Һ��Ũ�ȷֱ�Ϊc1��c2mol/L��������ΪVmL�����ǰ��Һ��ƽ���¶�Ϊt1�������Һ������¶�Ϊt2�����к��ȡ�H= kJ/mol����д���Ĵ���ʽ�������֣���
��1�����ν�������ý����Ƶ�ԭ����
��2����ͬһ�¶ȼƲ���������¶Ⱥ��ٲ�����������Һ�¶�ǰ����еIJ����ǣ�
��3��װ���д�С�ձ�����������ĭ���ϣ�����ֽ����������
��4����֪ˮ�ı�����Ϊ4.18J/��g?�棩��ϡ�����ϡ����������Һ��Ũ�ȷֱ�Ϊc1��c2mol/L��������ΪVmL�����ǰ��Һ��ƽ���¶�Ϊt1�������Һ������¶�Ϊt2�����к��ȡ�H=
���㣺�к��ȵIJⶨ
ר�⣺��ѧ��Ӧ�е������仯
��������1���к�ʵ��Ҫ��ֹ������ɢʧ���������ȵ������壻
��2��Ϊ�˼����ȷ������Ӧȫ����Ӧ������Ҫ�����¶ȼ��ϵ�����ˮ��ϴ�ɾ�������ϴ��Һ�������У�
��3��Ϊ��ֹ����ɢʧ�������ձ�����װ����ĭ���ϻ���ֽ����
��4���ȸ���Q=m?c?��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-
kJ/mol�������Ӧ�ȣ�
��2��Ϊ�˼����ȷ������Ӧȫ����Ӧ������Ҫ�����¶ȼ��ϵ�����ˮ��ϴ�ɾ�������ϴ��Һ�������У�
��3��Ϊ��ֹ����ɢʧ�������ձ�����װ����ĭ���ϻ���ֽ����
��4���ȸ���Q=m?c?��T���㷴Ӧ�ų���������Ȼ����ݡ�H=-
| Q |
| n |
���
�⣺��1����ʵ��Ҫ��ֹ������ɢʧ���������ȵ������壬�ʴ�Ϊ�������״��ȣ�������������ʧ��
��2��Ϊ�˼����ȷ������Ӧȫ����Ӧ������Ҫ�����¶ȼ��ϵ�����ˮ��ϴ�ɾ�������ϴ��Һ�������У��ʴ�Ϊ�����¶ȼ��ϵ�����ˮ��ϴ�ɾ���
��3��Ϊ��ֹ����ɢʧ�������ձ�����װ����ĭ���ϻ���ֽ�����ʴ�Ϊ����С������ʧ��
��4��ϡ�����ϡ����������Һ��Ũ�ȷֱ�Ϊc1��c2mol/L��������ΪVmL�������кͷ�Ӧ����ˮ�����ʵ���ΪV��10-3L��c1mol/L=
mol����Һ������Ϊ��2VmL��1g/ml=2Vg��
�¶ȱ仯ֵΪt2-t1��������
molˮ�ų�������ΪQ=m?c?��T=2Vg��4.18J/��g?�棩����t2-t1����=8.36��t2-t1����VJ����0.00836��t2-t1����VkJ��
����ʵ���õ��к��ȡ�H=-
=-
kJ/mol��
�ʴ�Ϊ��-
��
��2��Ϊ�˼����ȷ������Ӧȫ����Ӧ������Ҫ�����¶ȼ��ϵ�����ˮ��ϴ�ɾ�������ϴ��Һ�������У��ʴ�Ϊ�����¶ȼ��ϵ�����ˮ��ϴ�ɾ���
��3��Ϊ��ֹ����ɢʧ�������ձ�����װ����ĭ���ϻ���ֽ�����ʴ�Ϊ����С������ʧ��
��4��ϡ�����ϡ����������Һ��Ũ�ȷֱ�Ϊc1��c2mol/L��������ΪVmL�������кͷ�Ӧ����ˮ�����ʵ���ΪV��10-3L��c1mol/L=
| c1V |
| 1000 |
�¶ȱ仯ֵΪt2-t1��������
| c1V |
| 1000 |
����ʵ���õ��к��ȡ�H=-
| 0.00836(t2-t1)��VKJ | ||
|
| 8.36(t2-t1) |
| c1 |
�ʴ�Ϊ��-
| 8.36(t2-t1) |
| c1 |
������������Ҫ�����˲ⶨ�к���ʵ���ע��������к��ȵ��йؼ��㣬����ԭ���ǽ���Ĺؼ����Ѷ��еȣ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
��ͼ��ijЩʵ��IJ���װ�ã�������ע����ʵ���ܴﵽʵ��Ŀ���ǣ�������
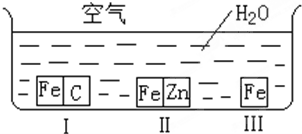
A�� ��ȥCO�����е�CO2���� |
B�� �������еĵ�;ƾ� |
C�� ��֤NH3���ܽ��� |
D�� �Ƚ�Na2CO3��NaHCO3�����ȶ��� |

 ��
��