��Ŀ����
7��������Һ�����ӵ����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������| A�� | 0.1 mol•L-1 NaHCO3��Һ��0.1 mol•L-1 NaOH��Һ�������ϣ�������Һ�У�c��Na+����c��CO32-����c��HCO32-����c��OH-�� | |
| B�� | 20 mL 0.1 mol•L-1 CH3COONa��Һ��10 mL 0.1 mol•L-1 HCl��Һ��Ϻ���Һ�����ԣ�������Һ�У�c��CH3COO-����c��Cl-����c��CH3COOH����c��H+�� | |
| C�� | �����£�pH=2��������pH=12�İ�ˮ�������ϣ�������Һ�У�c��Cl-��+c��H+����c��NH4+��+c��OH-�� | |
| D�� | 0.1 mol•L-1 CH3COOH��Һ��0.1 mol•L-1 NaOH��Һ�������ϣ�������Һ�У�c��OH-����c��H+��+c��CH3COOH�� |
���� A.0.1 mol•L-1 NaHCO3��Һ��0.1 mol•L-1 NaOH��Һ�������ϣ�����ǡ����ȫ��Ӧ����Na2CO3��̼�������ˮ�����Һ�ʼ��ԣ���ˮ��̶Ƚ�С��ˮ����Ҳ�������������ӣ�
B.20 mL 0.1 mol•L-1 CH3COONa��Һ��10 mL 0.1 mol•L-1 HCl��Һ��Ϻ���Һ�����ԣ���Һ������Ϊ�����ʵ���Ũ�ȵ�CH3COONa��NaCl��CH3COOH����Һ�����ԣ�˵���������̶ȴ��ڴ�����ˮ��̶ȣ����������̶Ƚ�С��
C�������£�pH=2������Ũ��С��pH=12�İ�ˮ�����ߵ������Ϻ�ˮ��ʣ�࣬��Һ�ʼ��ԣ�����Һ����Ȼ���ڵ���غ㣬���ݵ���غ��жϣ�
D.0.1 mol•L-1 CH3COOH��Һ��0.1 mol•L-1 NaOH��Һ�������ϣ�����ǡ����ȫ��Ӧ���ɴ����ƣ���Һ�д��������غ㣬���������غ��жϣ�
��� �⣺A.0.1 mol•L-1 NaHCO3��Һ��0.1 mol•L-1 NaOH��Һ�������ϣ�����ǡ����ȫ��Ӧ����Na2CO3��̼�������ˮ�����Һ�ʼ��ԣ���ˮ��̶Ƚ�С��ˮ����Ҳ�������������ӣ���������Ũ�ȴ�С˳����c��Na+����c��CO32-����c��OH-����c��HCO32-������A����
B.20 mL 0.1 mol•L-1 CH3COONa��Һ��10 mL 0.1 mol•L-1 HCl��Һ��Ϻ���Һ�����ԣ���Һ������Ϊ�����ʵ���Ũ�ȵ�CH3COONa��NaCl��CH3COOH����Һ�����ԣ�˵���������̶ȴ��ڴ�����ˮ��̶ȣ����������̶Ƚ�С����ϵ���غ������Ũ�ȴ�С˳���ǣ�c��CH3COO-����c��Cl-����c��CH3COOH����c��H+������B��ȷ��
C�������£�pH=2������Ũ��С��pH=12�İ�ˮ�����ߵ������Ϻ�ˮ��ʣ�࣬��Һ�ʼ��ԣ�c��H+����c��OH-������Һ����Ȼ���ڵ���غ㣬���ݵ���غ��c��Cl-����c��NH4+�������Ե�c��Cl-��+c��H+����c��NH4+��+c��OH-������C����
D.0.1 mol•L-1 CH3COOH��Һ��0.1 mol•L-1 NaOH��Һ�������ϣ�����ǡ����ȫ��Ӧ���ɴ����ƣ���Һ�д��������غ㣬���������غ��c��OH-��=c��H+��+c��CH3COOH������D����
��ѡB��
���� ���⿼������Ũ�ȴ�С�Ƚϣ�Ϊ��Ƶ���㣬���ؿ���ѧ�������ж���������ȷ��Һ�����ʼ��������ǽⱾ��ؼ����κε������Һ�ж����ڵ���غ㡢�����غ�������غ㣬��Щ�غ�����Һ����Լ�Ũ���أ���Ŀ�Ѷ��еȣ�

 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�| A�� | ������Cl2ȡ�� | B�� | ��ϩ��Cl2�ӳ� | ||
| C�� | ��Ȳ��HCl�ӳ� | D�� | �Ҵ���Ũ����ȡ�� |
| A�� | �٢� | B�� | �ڢ� | C�� | �� | D�� | �������� |
| A�� | �ڳ��³�ѹ��ΪҺ�� | B�� | ��ʹ����KMnO4��Һ��ɫ | ||
| C�� | �������ӳ��γɾ���ϩ | D�� | ��ʹ���CCl4��Һ��ɫ |
| A�� |  | B�� |  | C�� |  | D�� | 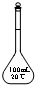 |
| A�� | Na2O��Na2O2�ȶ� | B�� | ��������ˮ��Ӧ | ||
| C�� | ������CO2��Ӧ | D�� | ���ǵ��͵ļ��������� |
 ��X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ�أ�����ԭ�ӵ�������������������Ӳ�����2����Z��Y������Z��W���γ�һ��WZ2�ͷ��ӣ�
��X����������Y�������ӵĵ��Ӳ�ṹ��ͬ��Ԫ��Z��W��Ϊ������Ԫ�أ�����ԭ�ӵ�������������������Ӳ�����2����Z��Y������Z��W���γ�һ��WZ2�ͷ��ӣ�