��Ŀ����
�����������ʵ��ó��Ľ�����ȷ���ǣ�������
| A����ij��Һ�м���ϡ���ᣬ����������ͨ�����ʯ��ˮ��ʯ��ˮ����ǣ�����Һһ������CO32- |
| B���ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ������Һһ������Na+ |
| C����ij��Һ�еμ�BaCl2��Һ������ɫ�������ٵμ�ϡHNO3�������ܽ⣬��ԭ��Һ��һ������SO42- |
| D����ij��Һ�еμ�KSCN ��Һ����Һ����ɫ���μ���ˮ����Һ�Ժ�ɫ������Һ��һ����Fe2+ |
���㣺���ʵļ���ͼ����ʵ�鷽�����
ר�⣺���ʼ��������
������A���ܹ�ʹ����ʯ��ˮ�����ڵ������ж�����̼�Ͷ�������ԭ��Һ�п��ܴ���̼������ӡ�����������ӡ�̼��������ӵȣ�
B����ɫ��Ӧ�Ļ���Ϊ��ɫ������Ϊ��ԭ�ӣ���Һ��һ�����������ӣ�����Ϊ���Ρ��������ƣ�
C���ܹ����Ȼ�����Ӧ���ɲ���������ij��������ӿ���Ϊ�����ӡ�SO42-���ӣ�
D��Fe2+����ˮ������������Fe3+����ʹKSCN��Һ��ʾ��ɫ��
B����ɫ��Ӧ�Ļ���Ϊ��ɫ������Ϊ��ԭ�ӣ���Һ��һ�����������ӣ�����Ϊ���Ρ��������ƣ�
C���ܹ����Ȼ�����Ӧ���ɲ���������ij��������ӿ���Ϊ�����ӡ�SO42-���ӣ�
D��Fe2+����ˮ������������Fe3+����ʹKSCN��Һ��ʾ��ɫ��
���
�⣺A����ij��Һ�м���ϡ���ᣬ����������ͨ�����ʯ��ˮ������ǣ��������������Ϊ������̼����������ԭ��Һ�п��ܴ���̼������ӡ�����������ӡ�̼��������ӵȣ����Բ�һ������CO32-����A����
B�������ӵļ��飺�ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ����һ�����������ӣ���B��ȷ��
C����ij��Һ�еμ�BaCl2��Һ������ɫ�������ٵμ�ϡHNO3�������ܽ⣬��ԭ��Һ���ܺ���Cl-����C����
D����ij��Һ�еμ�KSCN��Һ����Һ����ɫ���μ���ˮ����Һ�Ժ�ɫ��˵��Fe2+����������Fe3+������Һ��һ����Fe2+����D��ȷ��
��ѡBD��
B�������ӵļ��飺�ò�˿պȡ����ij��Һ������ɫ��Ӧ������ʻ�ɫ����һ�����������ӣ���B��ȷ��
C����ij��Һ�еμ�BaCl2��Һ������ɫ�������ٵμ�ϡHNO3�������ܽ⣬��ԭ��Һ���ܺ���Cl-����C����
D����ij��Һ�еμ�KSCN��Һ����Һ����ɫ���μ���ˮ����Һ�Ժ�ɫ��˵��Fe2+����������Fe3+������Һ��һ����Fe2+����D��ȷ��
��ѡBD��
���������⿼���˳������ӵļ��飬��Ŀ�Ѷ��еȣ�ע����ȷ�������ӵļ��鷽����ע������������������⣮

��ϰ��ϵ�д�
 �Ͻ�ƽСѧ��������ϵ�д�
�Ͻ�ƽСѧ��������ϵ�д�
�����Ŀ
��������У��γɹ��ۼ���ԭ�ӹ���ǣ�������
| A����ԭ�ӵ�2p�������ԭ�ӵ�1s��� |
| B����ԭ�ӵ�2p�������ԭ�ӵ�2p��� |
| C����ԭ�ӵ�3p�������ԭ�ӵ�1s��� |
| D����ԭ�ӵ�2p�������ԭ�ӵ�3p��� |
Ϊ��������ƿ��ɫ��Һ��HCl��AlCl3��Ba��NO3��2��NaCl��Na2CO3����λͬѧ��û�þƾ��ƣ���λͬѧ�������Լ���һλͬѧû�������κ��Լ������в�������һ�����ٵ��ǣ�������
| A���������˷�̪��Һ |
| B����������NaOH��Һ |
| C����������ʯ����Һ |
| D�����������κ��Լ� |
ԭ������Ϊ26��Fe����δ�ɶԵĵ�����Ϊ��������
| A��5 | B��4 | C��3 | D��2 |
���б��﷽ʽ��ȷ���ǣ�������
A����̬̼ԭ�ӵļ۵����Ų�ͼ��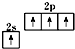 |
| B��HClO�Ľṹʽ��H-Cl-O |
| C����̬26Fe�ļ۵����Ų�ʽ��3d64s2 |
D����̬ͭԭ�ӵļ۵����Ų�ͼ��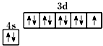 |
���и������У�����ԭ�Ӷ����������8���ӽṹ���ǣ�������
| A��BeCl2 |
| B��PCl3 |
| C��NH3 |
| D��PCl5 |


 �����أ���ԭ���ϵ���ԭ�ӿ������ϵ���ԭ���������ȩ�����ӳɷ�Ӧ�������۳ɸ߷��ӣ�
�����أ���ԭ���ϵ���ԭ�ӿ������ϵ���ԭ���������ȩ�����ӳɷ�Ӧ�������۳ɸ߷��ӣ� ��B�ķ�Ӧ������
��B�ķ�Ӧ������