��Ŀ����
13��ij��ѧ��ȤС��������ϡ�������ⶨ��������̼��Ƶ�������������1����������������ƽ����12g��ɡ������ļ����ǣ�
ϡ�ͣ�������24%�����ᣨ�ܶ�ԼΪ1.1g/cm3��ϡ�ͳ�240mL 7.3%��ϡ���ᣨ�ܶ�ԼΪ1.0g/cm3��������24%����������Ϊ66.4mL������ȷ��0.1��
��2��100g������������Ϊ7.3%��ϡ������12g������ǡ����ȫ��Ӧ�����ʲ���Ӧ����������̼��Ƶ�����������������ȷ��0.1%��
���� ��1��������Һϡ��ǰ�����ʵ��������������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ����ǡ����ȫ��Ӧ�����������������������̼��Ƶ�����������������
��� �⣺��1��������Һϡ��ǰ�����ʵ�������������24%����������Ϊx�ɵã�
24%��x��1.1g/cm3=240mL��1.0g/cm3��7.3%
x=66.4mL��
��2����μӷ�Ӧ��̼��Ƶ�����Ϊx��
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 73
x 100g��7.3%
$\frac{100}{x}$=$\frac{73}{100g��7.3%}$
x=10g
��������̼��Ƶ���������$\frac{10g}{12g}$��100%��83.3%
�ʴ�Ϊ����1��66.4mL��
��2��83.3%��
���� ���ݻ�ѧ����ʽ�ļ��㣬��ʹ�����ʵ���������Ϊ������������������ʵ����ʵ���������ת��Ϊ��������������ܴ��뻯ѧ����ʽ�ļ��㣮

��ϰ��ϵ�д�
�����Ŀ
3��ij�о�С����������ᾧ����Ʒ�ֽ������ⶨ��Ʒ�в��ᾧ��������������������ʲ����뷴Ӧ�������ᾧ�壨H2C2O4•2H2O ���IJ����������ʼ��±���
��1��ͼ1�Ǽ���װ�ã������˵ļ��ȷֽ���ᾧ��װ����c��
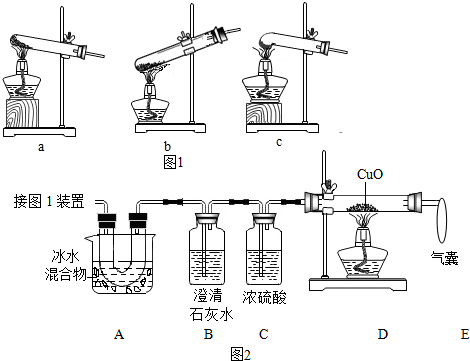
��2��ͼ2����֤�ȷֽ�����к� CO��CO2��װ�ã�
��װ��A����Ҫ�����dz�ȥ������������ֹ�Զ�����̼�ļ���������ţ�
�����ҵ��������ռ�δ��Ӧ��һ����̼����ֹ������Ⱦ��
��֤������CO2��������Bװ���ڵij���ʯ��ˮ����ǣ�B�з�Ӧ�Ļ�ѧ����ʽCO2+Ca��OH��2�TCaCO3��+H2O��
��֤������CO��������Dװ���к�ɫ�����죮
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�������������������·�������ȡһ������Ʒ��������װ�ý���ʵ�飬����װ��D��Ӧǰ���������ɴ˼������ʵ������ʵ��ֵƫ�ͣ��ų������Ͳ������أ������ԭ��һ����̼û��ȫ��������ͭ��Ӧ����дһ�����ɣ�
��4����ȡ17.5g���ᾧ����Ʒ����50.00g��Һ����������ϡ���ᣬȻ��μ�KMnO4��Һ����KMnO47.9�ˣ�ǡ�÷�Ӧ��ȫ������֪��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��
�������Ʒ�в��ᾧ�壨H2C2O4•2H2O ����������������д��������̣�
[�й����ʵ���Է���������Mr��H2C2O4��=90��Mr��H2C2O4•2H2O��=126��Mr��KMnO4��=158]��
| �� �� | �� �� | �� �� �� �� | �� �� �� Ӧ |
| 101�桫102�� | 150�桫160�� ���� | 100.1��ֽ��ˮ��175��ֽ��CO2��CO��H2O | �� Ca��OH��2��Ӧ������ɫ������CaC2O4�� |

��2��ͼ2����֤�ȷֽ�����к� CO��CO2��װ�ã�
��װ��A����Ҫ�����dz�ȥ������������ֹ�Զ�����̼�ļ���������ţ�
�����ҵ��������ռ�δ��Ӧ��һ����̼����ֹ������Ⱦ��
��֤������CO2��������Bװ���ڵij���ʯ��ˮ����ǣ�B�з�Ӧ�Ļ�ѧ����ʽCO2+Ca��OH��2�TCaCO3��+H2O��
��֤������CO��������Dװ���к�ɫ�����죮
��3��Ϊ�ⶨ��Ʒ�в��ᾧ�������������������·�������ȡһ������Ʒ��������װ�ý���ʵ�飬����װ��D��Ӧǰ���������ɴ˼������ʵ������ʵ��ֵƫ�ͣ��ų������Ͳ������أ������ԭ��һ����̼û��ȫ��������ͭ��Ӧ����дһ�����ɣ�
��4����ȡ17.5g���ᾧ����Ʒ����50.00g��Һ����������ϡ���ᣬȻ��μ�KMnO4��Һ����KMnO47.9�ˣ�ǡ�÷�Ӧ��ȫ������֪��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��
�������Ʒ�в��ᾧ�壨H2C2O4•2H2O ����������������д��������̣�
[�й����ʵ���Է���������Mr��H2C2O4��=90��Mr��H2C2O4•2H2O��=126��Mr��KMnO4��=158]��
4�������������ڴ�������ǣ�������
| A�� | ˮ | B�� | ���� | C�� | ��� | D�� | ���� |
1��2015��3��22���ǵڶ�ʮ���硰����ˮ�ա������Ϲ�ȷ����������������ǡ�ˮ��ɳ�����չ��������ˮ��˵��������ǣ�������
| A�� | ���ó����Ǿ���ˮ�̶���ߵķ��� | B�� | �÷���ˮ���Լ���Ӳˮ����ˮ | ||
| C�� | ����ʹ��ũҩ�����ˮ����Ⱦ | D�� | ��ˮ���������Ȼ��ƺ��������� |
18�����н����У����������ǿ���ǣ�������
| A�� | Mg | B�� | Cu | C�� | Fe | D�� | Na |
2������ÿ�������У���ѧʽ����������ƥ���һ����ǣ�������
| A�� | NaOH ��� | B�� | Ca��OH��2 ��ʯ�� | C�� | NaHCO3 С�մ� | D�� | CaCO3ʯ��ʯ |
 ��1���û�ѧ������գ�
��1���û�ѧ������գ�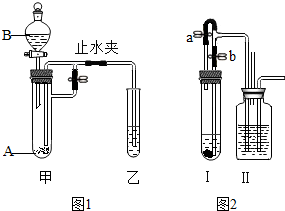 ij��ѧС��ͬѧ����ͼʾװ�ý���ʵ�飨ͼ�й̶�װ��ʡ�ԣ�������װ�ü��Թ���ʢ�й����ĩA����Һ©����ʢ��������ҺB��
ij��ѧС��ͬѧ����ͼʾװ�ý���ʵ�飨ͼ�й̶�װ��ʡ�ԣ�������װ�ü��Թ���ʢ�й����ĩA����Һ©����ʢ��������ҺB��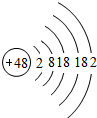 ������ʳƷ�ල�����ֹ������������һ����ʳƷ������ݣ��ڳ���18����������Ʒ�У���8���β�Ʒ�ӳ��꣮��������ɫ�н�������Ľ��������ڳ�ʪ�����л���������ʧȥ����������ʱ�����γ�������㣬�����Ӻ�ǿ�����ӵ��ܽ�ȶ���С�����������ᣬ�������ڼ�����Ķ����ϲ��ϻش�һ�����⣺
������ʳƷ�ල�����ֹ������������һ����ʳƷ������ݣ��ڳ���18����������Ʒ�У���8���β�Ʒ�ӳ��꣮��������ɫ�н�������Ľ��������ڳ�ʪ�����л���������ʧȥ����������ʱ�����γ�������㣬�����Ӻ�ǿ�����ӵ��ܽ�ȶ���С�����������ᣬ�������ڼ�����Ķ����ϲ��ϻش�һ�����⣺ ���û�ѧʽ����ʽ�С�
���û�ѧʽ����ʽ�С�