��Ŀ����
11��Сǩͬѧ��ȷ����ҵ��Ʒ����صĺ����������̽���������ʵ�飺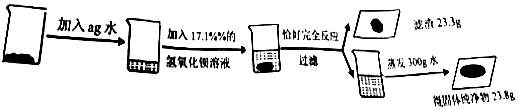
��1����д��������Ӧ�Ļ�ѧ����ʽK2SO4+Ba��OH��2=BaSO4��+2KOH��
��2���г��������ع���������x���ı���ʽΪ$\frac{174}{233}=\frac{x}{23.3g}$��
��3����ҵ��Ʒ������صĺ���Ϊ48.9%��
��4������ˮ��������217.1g��
��5���ô��������Ʒ������������Һ��Ӧ����������119t�������Ʒ������Ϊ378.25t��
���� ��1�����������������������Ӧ�������ᱵ�������������ؽ��з�����
��2�����ݻ�ѧ����ʽ�и����ʵ������ȷ������
��3�����ݻ�ѧ����ʽ����ϳ��������������������ص��������ɽ��
��4�����������غ㶨�ɼ���������Һ��������
��6�����ݻ�ѧ����ʽ����ϳ���������������������������Ʒ���������ɽ��
��� �⣺��1����Ϊ�����������������Ӧ�������ᱵ�������������أ����䷴Ӧ�Ļ�ѧ����ʽΪ��K2SO4+Ba��OH��2=BaSO4��+2KOH��
��2��������ص�����Ϊx��
K2SO4+Ba��OH��2=BaSO4��+2KOH
174 233
x 23.3g
��$\frac{174}{233}=\frac{x}{23.3g}$�����x=17.4g��
��3����KOH������Ϊy��
K2SO4+Ba��OH��2=BaSO4��+2KOH
233 56
23.3g y
��$\frac{233}{56}=\frac{23.3g}{y}$��y=5.6g��
�ʹ�ҵ��Ʒ��������������Ƶ�����Ϊ��23.8g-5.6g=18.2g��
��ҵ��Ʒ������صĺ���Ϊ$\frac{17.4g}{17.4g+18.2g}$��100%��48.9%��
��4����Ba��OH��2������Ϊz��
K2SO4+Ba��OH��2=BaSO4��+2KOH
171 233
z 23.3g
��$\frac{171}{233}=\frac{z}{23.3g}$�����z=17.1g��
����ˮ�������ǣ�300g-��$\frac{17.1g}{17.1%}$-17.1g��=217.1g
��5��������ص�����Ϊx��
K2SO4+Ba��OH��2=BaSO4��+2KOH
174 112
x 119t
��$\frac{174}{112}=\frac{x}{119t}$�����x=184.875t��
�ʴ��������Ʒ������������Һ��Ӧ����������119t�������Ʒ������Ϊ184.875t�£�$\frac{17.4g}{17.4g+18.2g}$��100%��=378.25t��
�ʴ�Ϊ��
��1��K2SO4+Ba��OH��2=BaSO4��+2KOH����2��$\frac{174}{233}=\frac{x}{23.3g}$����3��48.9%����4��217.1����5��378.25t��
���� ���⿼�����Ȼ�����̼���ƵĻ�ѧ���ʣ�ͬʱ�����˻�ѧ����ʽ����д�Լ����ݻ�ѧ����ʽ������Һ������������Ҫ��ѧ���������ճ������ʵĻ�ѧ�����Լ���صĻ�ѧ����ʽ��

| A�� | Ũ���᳨�ڷ��ã��ڿ������γɡ������� | |
| B�� | ��ʳ�׳�ȥˮ���е�ˮ�� | |
| C�� | ������̼��������� | |
| D�� | ��¯���� |
| A�� | H+ Cl- NO3- Ba2+ | B�� | Na+ Cu2+ Cl- NO3- | ||
| C�� | Fe3+ K+ Cl- SO42- | D�� | Ba2+ NO3- Na+ Cl- |
| �¶ȣ��棩 | ʹ�õ����� | ʹ�õ��ܼ� | �۲쵽��ʵ���� |
| 20 | ��2g | �ƾ�10g | ȫ���ܽ� |
| 20 | ��2g | ˮ10g | �����ܽ� |
| A�� | �����ܽ������Ĵ�С�����ʵ������й� | |
| B�� | �����ܽ������Ĵ�С���ܼ��������й� | |
| C�� | �����ܽ������Ĵ�С���¶��й� | |
| D�� | �����ܽ������Ĵ�С�����ʵ������й� |
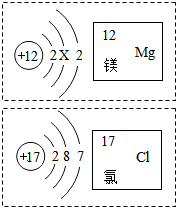 ��ͼ��þ��������Ԫ�ص��й���Ϣ��������˵��������ǣ�������
��ͼ��þ��������Ԫ�ص��й���Ϣ��������˵��������ǣ�������| A�� | þԭ�ӽṹͼ��X=8 | |
| B�� | ��Ԫ�ص�ԭ������Ϊ17 | |
| C�� | þԪ������Ԫ����ʵ�������������������ͬ | |
| D�� | þ������ɻ�����Ļ�ѧʽΪMgCl2 |
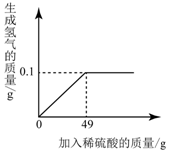 С��ͬѧ��ⶨijCu-Zn�Ͻ���ͭ������������ȡһ�����Ͻ��ĩ������������һ����������������ϡ���ᣬ����ϡ����������������������ϵ��ͼ��ʾ����������з��������㣺
С��ͬѧ��ⶨijCu-Zn�Ͻ���ͭ������������ȡһ�����Ͻ��ĩ������������һ����������������ϡ���ᣬ����ϡ����������������������ϵ��ͼ��ʾ����������з��������㣺 С��ͬѧ��������ͼ��ʾA��B���ֹ������ʵ���Һ�����ߣ�
С��ͬѧ��������ͼ��ʾA��B���ֹ������ʵ���Һ�����ߣ�