��Ŀ����
12��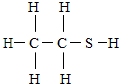 ������2000��漪��˹�����¼����¼����������ʣ������н�������ڷ�֮һ������ʱ�����ζ�Ϳ��ᵽ��ͨ��������Һ��ú��������ζָʾ�������Ľṹʽ��ͼ����ش��������⣺
������2000��漪��˹�����¼����¼����������ʣ������н�������ڷ�֮һ������ʱ�����ζ�Ϳ��ᵽ��ͨ��������Һ��ú��������ζָʾ�������Ľṹʽ��ͼ����ش��������⣺��1��д�����Ļ�ѧʽC2H6S��������Է�������Ϊ62��������
����������������
��2��������̼���⡢��ԭ�Ӹ�������2��6��1��
��3�������п�ȼ�ԣ���д������ȫȼ�յĻ�ѧ��Ӧ����ʽ2C2H6S+9O2$\frac{\underline{\;��ȼ\;}}{\;}$4CO2+2SO2+6H2O��
���� ��1�����ݷ��ӽṹģ�ͷ�������ѧʽ��Ȼ����ݻ�ѧʽ��������
��2�����ݷ��ӽṹ��������
��3�����ݻ�ѧ����ʽ��д�����������
��� �⣺��1���ɷ��ӽṹģ�Ϳ�֪��1������������2��̼ԭ�ӡ�6����ԭ�Ӻ�1����ԭ�ӹ��ɵģ��ʻ�ѧʽΪC2H6S��������Է�������Ϊ12��2+1��6+32=62������һ�ֺ�̼Ԫ�صĻ���������л�����C2H6S��62���л��
��2��1������������2��̼ԭ�ӡ�6����ԭ�Ӻ�1����ԭ�ӹ��ɵģ�����������̼���⡢��ԭ�Ӹ�������2��6��1�����2��6��1��
��3���������غ㶨�ɿ�֪��������ȫȼ������ˮ��������̼�Ͷ��������2C2H6S+9O2$\frac{\underline{\;��ȼ\;}}{\;}$ 4CO2+2SO2+6H2O��
���� �����ѶȲ�����ͬѧ�ǽ������Ϣ��������û�ѧʽ�ĺ������йؼ�����з������⡢��������������

��ϰ��ϵ�д�
�����Ŀ
3������˵���У�������ǣ�������
| A�� | �������ƺ��������ƣ��������ڸ����������� | |
| B�� | �������ú�̼��Ƶ�ҩ�����ȿɲ����ֿ�����θ����� | |
| C�� | ��ȥ�����в��������ʣ���Ҫ�ȰѴ����ܽ⣬Ȼ����ˡ����� | |
| D�� | ��ҵ���ó���������ʱ�����������Ҫ�ɷ�Fe2O3�����˻�ԭ��Ӧ |
20�������ȴʣ�������ϵİ�ȫ����������������ʳƷ��ȫҪ����ǣ�������
| A�� | �����㺣Ϻ��������ȩ��Һ���� | |
| B�� | �����������Ӽ���ʹ���ٳ� | |
| C�� | Ϊ��ʹ��Ҷ������������ͭ��Һ������Ҷ | |
| D�� | ���������С�մ������ɼ� |
17�����и������ʰ����ʡ��������˳�����е�һ���ǣ�������
| A�� | C CaO NaHSO4 | B�� | H2 H2O NaOH | C�� | O2 NaOH H2SO4 | D�� | Fe CO2 H3PO4 |
 �Ķ�������ն��ģ�
�Ķ�������ն��ģ� ����ģ�ͺ���֪ʶ�����ǻ�ѧѧϰ����Ҫ��ѧϰ�������ͬѧ�������ֹ����������֪ʶ���磬��ͼ��ʾ������ͼʾ�ش��������⣺
����ģ�ͺ���֪ʶ�����ǻ�ѧѧϰ����Ҫ��ѧϰ�������ͬѧ�������ֹ����������֪ʶ���磬��ͼ��ʾ������ͼʾ�ش��������⣺
