��Ŀ����
16���û�ѧ֪ʶ�����������⣺��1������Ҫ���в��ṩ��ѵ����ϴ�����������Ŀ�������ƺͼ������ϴ���ʹ�ã����ٰ�ɫ��Ⱦ��
��2����ˮʱ��ˮ�м��������������Ƕ�����������ʹ���ʳ�����
��3����ˮǹ��ˮ����ԭ����ʹ�¶Ƚ�����ȼ����Ż�����£�
��4������������ȷֽ��ʣ������������ԭ��Ӧ�������С������Ϊ��Ӧ�����ɵ������ݳ���
��5����ϴ�ྫ��ϴȥ�·��ϵ����ۣ�ϴ�ྫ���黯���ã�
���� ��1�����ݷ������ϻ���ɰ�ɫ��Ⱦ�����з������
��2�����������ھ�ˮ�е����ý��з������
��3���������ԭ���������������ȼ��ڸ����������������ʹ�¶Ƚ�����ȼ����Ż�����£��ݴ˽��������з������
��4�����������غ㶨�ɣ���ϸ�����ؼ��ȷֽ���������ء��������̺����������з������
��5������ϴ�ྫ�����黯���ã����з������
��� �⣺��1������Ҫ���в��ṩ��ѵ����ϴ�����������Ŀ�������ƺͼ������ϴ���ʹ�ã����ٰ�ɫ��Ⱦ��
��2�������dz��õľ�ˮ������������������ˮ�����ɵĽ�״������ʵ�������ʹ������ˮ�е����ʳ�����
��3����ˮǹ��ˮ�����������ʹ�¶Ƚ�����ȼ����Ż�����µ����ԭ����
��4��������ؼ��ȷֽ���������ء��������̺���������Ӧ�������ݳ�����ʣ������������ԭ��Ӧ�������С��
��5����ϴ�ྫ��ϴȥ�·��ϵ����ۣ�����Ϊϴ�ྫ�����黯���ã��ܽ�����͵η�ɢ��ϸС���͵���ˮ���ߣ�
�ʴ�Ϊ����1�����ƺͼ������ϴ���ʹ�ã����ٰ�ɫ��Ⱦ����2��������������ʹ���ʳ�������3��ʹ�¶Ƚ�����ȼ����Ż�����£���4����Ӧ�����ɵ������ݳ�����5���黯��
���� �����ѶȲ������ռ��ٰ�ɫ��Ⱦ�Ĵ�ʩ�����ԭ���������غ㶨�ɡ��黯���õ�����ȷ�����Ĺؼ���

 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�| A�� | NaCl NaOH NaNO3 | B�� | NaOH H2SO4 KNO3 | ||
| C�� | HCl NaCl Na2SO4 | D�� | Na2SO4 H2SO4 CuSO4 |
��1������������Ϻ��֣��ٽ����ڵ�ҩ���θ�д̼����ã�����ǵ���Ҫ�ɷ��ǵ��ۣ�
��2�������ҵ����ĥ�ɷ�ĩ���þƾ�ϴȥɫ�أ���������ʵ�飺
| �Թܱ�� | ʵ����� | ˮԡ�¶� | ˮԡʱ�� | �����Լ� | ���� | |
| 1�� | ���ҷ�ĩ0.1�� | ��Һ2���� | 37�� | 10���� | ��Һ2�� | �� |
| 2�� | ���ҷ�ĩ0.1�� | θҺ2���� | 37�� | 10���� | ��Һ2�� | ����ɫ |
| 3�� | ���ҷ�ĩ0.1�� | ��ˮ2���� | 37�� | 10���� | ��Һ2�� | ����ɫ |
��1��1���Թܵ������Dz�����ɫ��
��2��ֱ�Ӽ��齺�ҷ�ĩ�Ƿ��е��۵���3���Թܣ������Թܱ�ţ�
��3������ð�塱�Ľ�����ǵ������DZ��⽺���ڵ�ҩ���θ�����̼����ã�
| ��ʵ | ���� | |
| A | �����ͺ�����ѧ�������� | ��ԭ�Ӻͺ�ԭ��������������ͬ |
| B | �Ȼ��ƹ��岻���� | ��Ϊû�д�������� |
| C | ������ˮ���ױ�ѹ�� | �����ȴ�������Ӽ���� |
| D | ���ʵ��������� | ���Ӽ�ļ�����¶ȵĸı���ı� |
| A�� | A | B�� | B | C�� | C | D�� | D |
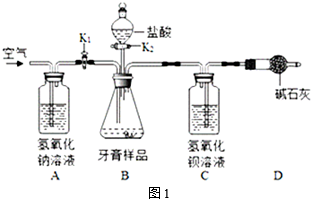
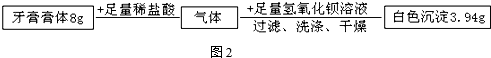
 �緹���������г��õĴ��ߣ���ͼ�ǵ緹��ʾ��ͼ
�緹���������г��õĴ��ߣ���ͼ�ǵ緹��ʾ��ͼ ��a=8ʱ���������������� ���ԭ�ӡ����������ӡ��������ӡ�����
��a=8ʱ���������������� ���ԭ�ӡ����������ӡ��������ӡ�����