��Ŀ����
7����1����ͼ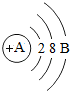 Ϊij���ӵĽṹʾ��ͼ����A-B=10ʱ����������ԭ�ӣ���ԭ�ӡ������ӻ������ӣ�������Na���ѧ�����B=8ʱ���������Ӵ�1����λ������ɣ�������ӵķ�����K+���������Ӵ�1����λ�ĸ���ɣ�������ӵķ�����Cl-��
Ϊij���ӵĽṹʾ��ͼ����A-B=10ʱ����������ԭ�ӣ���ԭ�ӡ������ӻ������ӣ�������Na���ѧ�����B=8ʱ���������Ӵ�1����λ������ɣ�������ӵķ�����K+���������Ӵ�1����λ�ĸ���ɣ�������ӵķ�����Cl-����2�������Ԫ�����ڱ���һ���֣�������Ԫ�ؾ�λ�ڵ������ڣ����ǵı�����������������ͬ������̼λ��ͬ�壬��ѧ�������ƣ�������������ռ���Һ��Ӧ�Ļ�ѧ����ʽΪSiO2+2NaOH=Na2SiO3+H2O
| 14 Si 28.09 | 15 P 30.97 | 16 S 32.06 | 17 Cl 35.45 |
���� ��1���������Ľṹ�ͺ�������������������Ŀ�Ĺ�ϵ�����ش��йص����⣮
��2��Ԫ���������������˵��������ͬ��һ��ԭ�ӵ��ܳƣ�����Ԫ��������������������˵��������ԭ�ӵĵ��Ӳ������������������ݶ�����̼���Һ�ķ�Ӧд�������������ռ���Һ��Ӧ�Ļ�ѧ����ʽ��
��� �⣺��1����ij���ӵĽṹʾ��ͼ����x-y=10ʱ��x=2+8+y��������=�����������Ϊԭ�ӣ�������ԭ�ӡ���ԭ�ӵȣ����ŷֱ�Ϊ��Na��S�ȣ���Y=8ʱ�������Ӵ���1��λ������ɣ���ԭ��ʧȥ1���ӵõ��ģ���x=2+8+8+1=19��Ϊ�����ӣ������ӷ���Ϊ��K+���������Ӵ�1����λ�ĸ���ɣ�x=2+8+8-1=17Ϊ�����ӣ������ӷ���Ϊ��Cl-��
��2��������Ԫ�ص�ԭ�������ֱ�Ϊ14��15��16��17���������������Ӳ㣬��λ�ڵ������ڣ����ǵı�����������������ͬ������̼λ��ͬ�壬��ѧ�������ƣ�������������ռ���Һ��Ӧ���ɹ����ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ��2NaOH+SiO2�TNa2SiO3+H2O��
�ʴ�Ϊ����1��ԭ�ӣ�Na��S���ȣ�K+��Cl-����2��������������ͬ��SiO2+2NaOH=Na2SiO3+H2O��
���� �����ѶȲ�����ѧ�������ӽṹʾ��ͼ������������⣬��ȷ�����к����������ͺ��������֮��Ĺ�ϵ�ǽ���Ĺؼ���

 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д�| ��Ӧǰ | ��Ӧ�� | ||
| ʵ���� ������ | �ձ���ϡ��������� | ʯ��ʯ��Ʒ������ | �ձ������л��������� |
| 150g | 15g | 160.6g | |
��1���÷�Ӧ�����ɶ�����̼������Ϊ4.4g��
��2����ʯ��ʯ��Ʒ��̼��Ƶ�������������ȷ��0.1%����
| A�� | ����ϩ���ϳ��Ͳ����������л��ϳɲ��� | |
| B�� | �ƹ���������ͼ�������ֲ��۸˵��Ƴ����������Һ��ʯ���� | |
| C�� | �Ϻ�������ܶ�չ�ݲ��ù��ת��װ�ã����ֵ���̼�����õ����� | |
| D�� | ���ö�����̼��ԭ�Ϻϳɿɽ������������ڼ��ٰ�ɫ��Ⱦ |
| A�� |  | B�� |  | C�� |  | D�� |  |
 ˮ����������������������أ�
ˮ����������������������أ� ijԪ��ԭ�ӵĽṹʾ��ͼ��ͼ����Ԫ��ԭ�Ӻ�����11 �����ӣ������ڽ���Ԫ�أ��ڻ�ѧ��Ӧ����ʧȥ�����γ������ӣ���������������Ԫ��ԭ���γɵ����ӷ���ΪNa+��������Ԫ���γɵĻ�����Ļ�ѧʽΪNa2S��
ijԪ��ԭ�ӵĽṹʾ��ͼ��ͼ����Ԫ��ԭ�Ӻ�����11 �����ӣ������ڽ���Ԫ�أ��ڻ�ѧ��Ӧ����ʧȥ�����γ������ӣ���������������Ԫ��ԭ���γɵ����ӷ���ΪNa+��������Ԫ���γɵĻ�����Ļ�ѧʽΪNa2S��