��Ŀ����
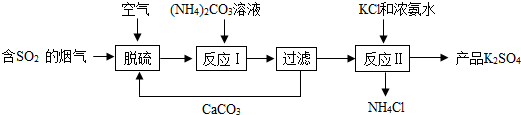
10�����������г��õĽ�������ͼ1��ij��ȡůƬ�����װ��ͼƬ���á�ȡůƬ���з��ȼ���Ҫ�ɷ������ۡ��Ȼ��ơ�ˮ������̿�ȣ��䷢��������������ʱ����ȵ�ԭ������1�����ȼ���Ӵ��������Żᷢ�ȣ�ԭ������Ҫ��������ˮ��ͬ���òŻ����⣮
��2����ȡůƬ���е��Ȼ��������Ǣڣ�����ţ�
������ˮ���� �ڼӿ������� ��û���κ���;
��3����ѧ��ȤС��ļס��Ҷ�λͬѧ�ԡ�ȡůƬ���ijɷֲ�������Ȥ��
��ͬѧ�ⶨ�µġ�ȡůƬ�������۵ĺ�����ȡ5.7g��ȡůƬ����Ʒ��Ͷ��װ��10.0gϡ���ᣨ���������ձ����ձ�����10.0g���У��ڻ�ѧ��Ӧ�����ж��ձ���������γ��������ݼ�¼���±���
| ��Ӧʱ�� | t0 | t1 | t2 | t3 | t4 |
| �ձ���ҩƷ����/g | 25.7 | 25.6 | 25.5 | 25.5 | m |
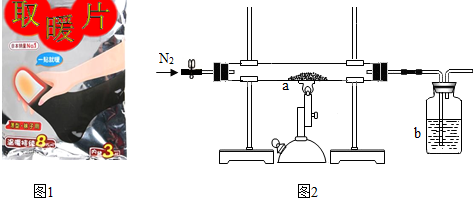
����ͬѧ�ⶨһ���ѱ��ʡ�ȡůƬ����Fe2O3������������ȡ10g�ѱ��ʡ�ȡůƬ����Ʒ�������ľ̿�ۻ�ϣ���ͼ2��ʾװ�����ʵ�飮
��ʵ���м���ǰҪ����ͨ��һ��ʱ�䵪�����������ž�װ���ڵĿ�����ֹͣ���Ⱥ�Ҫ����ͨ��һ��ʱ�䵪��������ᵼ�²ⶨ�Ľ��ƫС���ƫ����ƫС������
�ڳ�ַ�Ӧ����ͬѧ�ⶨb�е��Լ�������3.3g��b�е��Լ�������������Һ��������Ӧ�Ļ�ѧ����ʽΪCO2+2NaOH�TNa2CO3+H2O��
�������ͬѧ����ѱ��ʵġ�ȡůƬ����Ʒ��Fe2O3������������
���� ��1��������������������ǣ���2�������������Ӱ�����ؿ��ǣ���3���ٸ��ݱ��������жϷ�Ӧ�Ƿ���ȫ��Ӧ���ڸ��������ļ�������������������������������������������μӷ�Ӧ�������������ٸ���ͨ�뵪�������ûش��⿼�ǣ��ڱ�����Ҫ������b�е��Լ������������������ɶ�����̼������������b���Լ����������ն�����̼�ģ����Կ����ü���Һ���۸���b�е��Լ�������3.3g�Ƕ�����̼�����������ݶ�����̼�����������������������������������������������Ʒ�������ɣ�
��� �⣺��1������������������������ˮ��ͬ���õĽ����
��2�������ηֵ��������ܴٽ������⣬�ӿ������ٶȣ�
��3���ٸ��������ļ�������������������������ͼʾ���ݿ�֪����t2�Ѿ���Ӧ���ˣ�����t4�ձ���ҩƷ�����Dz���ģ�����25.5�ˣ�
�ڸ��������ļ�����������������������25.7g-25.5g=0.2g����Ҫ����0.2g������Ҫ��������ΪX��
Fe+2HCl�TFeCl2+H2��
56 2
X 0.2g
���ݣ�$\frac{56}{x}=\frac{2}{0.2g}$
���X=5.6g��
�ټ���ǰҪ����ͨ��һ��ʱ�䵪����Ϊ���ž�װ���ڵĿ����������������������ľ̿��Ӧ�����ɶ�����̼�������ֹͣ���Ⱥ�Ҫ����ͨ��һ��ʱ�䵪������Ϊ�˰ѷ�Ӧ���ɵĶ�����̼������bװ���ڣ������в��ֶ�����̼������װ���ڣ�ʹ������̼�����٣��������������������٣����ƫС��
�ڱ�����Ҫ������b�е��Լ������������������ɶ�����̼�����������ݶ�����̼�����������������������������b���Լ����������ն�����̼�ģ����Կ����ü���Һ��������������Һ����Ӧ�����������ƺͶ�����̼����������̼���ƺ�ˮ���ù۲취��ƽ���ɣ�
�۸���b�е��Լ�������3.3g�Ƕ�����̼����������Ҫ����3.3g������̼��Ҫ�μӷ�Ӧ��������������ΪY��
2Fe2O3+3C$\frac{\underline{\;����\;}}{\;}$4Fe+3CO2
320 132
Y 3.3g
���ݣ�$\frac{320}{y}=\frac{132}{3.3g}$
���Y=8g���ѱ��ʵġ�ȡůƬ����Ʒ��Fe2O3������������$\frac{8g}{10g}��100%$=80%��
�ʴ�Ϊ����1��������ˮ����2���ڣ���3����25.5����5.6�����ž�װ���ڵĿ�����ƫС�����������ƣ�CO2+2NaOH�TNa2CO3+H2O������Ʒ��Fe2O3����������80%��
���� �����ؼ���֪���������ᷴӦʱ���������ļ������������������������ٸ��������������������������̼����������Ӧʱ����b�����������������ɵĶ�����̼�����������ݶ�����̼���������������������������������������

| A�� | 84��85��79 | B�� | 1��1��1 | C�� | 23��24��18 | D�� | 1��1��5 |
 С���ǵ���ѧϰ�������˳���Ӧ��ʱ����ʦ��˵��һ�㲻����K��Ca��Na�Ȼ��ý���������Һ�����û���Ӧ����Ϊ��Щ������ֱ�Ӻ�ˮ������Ӧ���ɶ�Ӧ�ļ��������С�������������£�
С���ǵ���ѧϰ�������˳���Ӧ��ʱ����ʦ��˵��һ�㲻����K��Ca��Na�Ȼ��ý���������Һ�����û���Ӧ����Ϊ��Щ������ֱ�Ӻ�ˮ������Ӧ���ɶ�Ӧ�ļ��������С�������������£������Ͽ�Ƭ����
��1��Na����ˮ���ҷ�Ӧ��2Na+2H2O�T2NaOH+H2����
��2��þ����ˮ��Ӧ�������ͷ�ˮ��Ӧ���ң�Mg+2H2O�TMg��OH��2+H2����
��3��Al�ͷ�ˮ��Ӧ�dz�������2Al+6H2O�T2Al��OH��3+3H2����
�����������У����ܵó�������ˮ��Ӧ�������Ͳ����кβ�ͬ������֮����
��1����ͬ�㣺�����ɼ��������
��2����ͬ�㣺��Ӧ�ľ��ҳ̶Ȳ�ͬ��
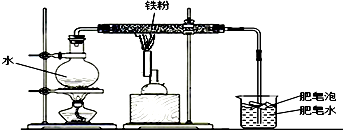
��С���Դ˷dz����棬����ʦ�İ����£�������������ϲ����������ڸ��������º�ˮ�����ķ�Ӧ��װ������ͼ��ʾ��
�����Ͽ�Ƭ����
A����������������ȶ���˳����FeO����ɫ����Fe3O4��Fe2O3����Fe3O4�д��ԣ�
B��Fe2O3�ֽ��¶���1400�����ϣ�Fe3O4�ֽ��¶���1538�����ϣ�
C��Fe��OH��2��һ�ְ�ɫ���壬�ڿ����ܿ��ɻ���ɫ�����ձ�Ϊ���ɫ��
ʵ�����2���Ӻ���ȼ�ŵ�ľ����ȼ�����ݣ����Թ۲쵽һ�Ż��森ͬʱ���ֲ������ڻҺ�ɫ�����Ⱥ��ȣ����ձ�ɺ�ɫ���壬����ʵ�����10�������ң�
��1������Ϊ�÷�ӦΪʲôҪ�ڸ����²��ܽ��У���֤���������������������ķֽ⣻
��2������IJ�����˵���÷�Ӧ�������������ڵ�ȼ������ǰ��һ��Ҫ���鴿�ȣ�
��3��ʵ������������е�ʵ�����Ӧ�����ȳ����ܣ���Ϩ��ƾ��ƣ���������Ŀ���Ƿ�ֹˮ����ˮ������ը���Թܣ�
��ͨ�����е�ʵ������ҶԷ�Ӧ�������в����ĺ�ɫ���ʵijɷֲ������£�
1��Fe��OH��3��2��Fe��OH��2��3��FeO��4��Fe2O3��5��Fe3O4��6��Fe�����ɶ��
��ͬѧ��Ϊ������1��2��4���Դ����������������������������������������ȶ��Բ
�����������룬ͬѧ������������ʵ�飺
| ʵ�鷽�� | ʵ������ | |
| �� | ������������ɫ��ĩ | ��ɫ��ĩ�ɱ�����ȫ������ |
| �� | ȡ������ɫ��ĩ����ϡ������ | ������ |
���ݼס�����ͬѧ��ʵ�飬�������ų�����3��5����������������������������������ϡ���ᷴӦ�������壻
Ϊ��һ��ȷ�ϴ˺�ɫ���ʣ�ͬѧ���ٴν�����ʵ�飺
| ʵ�鷽�� | ʵ������ | ���ۻ�ѧ����ʽ |
| 1��ȡʵ���ĺ�ɫ��ĩ������������A��Һ������ʹ��Ӧ��֣� | ��ɫ���ʱ����к�ɫ���ʣ���Һ��ɫ���ֽ�dz����ɫ���ձ��ײ����н϶��ɫ���� | Fe+CuSO4=FeSO4+Cu |
| 2�����ˡ�ϴ�ӡ�������ô������� | ��ɫ���ʿɱ�����ȫ�����������º�ɫ���� | �������������������� |
��1���������в����ĺ�ɫ����ӦΪ̼�����ۺ�ˮ�����ڸ����·�Ӧ�Ļ�ѧ����ʽΪ��3Fe+4H2O$\frac{\underline{\;����\;}}{\;}$Fe3O4+4H2�������û���Ӧ��
��2��ʵ��1�е�A��ҺΪ����ͭ��Һ����A��Һ��Ŀ������֤��ɫ�����к�������
������ͬѧ�ö����ķ���ȷ��ʵ��������к�ɫ���ʵ���ɣ��������£�
��5.6gFe�ۺ�������H2O��������ַ�Ӧ�õ�7.2g��ɫ���壬�ٽ��˺�ɫ�������150g����ϡ�����м�����Ӧ����ַ�Ӧ�Ƶ��ձ�����Һ����Ϊ157.15g��������������ݼ����7.2g��ɫ���ʵ���ɣ�ȷ֤����̽�����ۣ���д��������̣�
������˼���ۣ�
��1�����������ֿ��Ծ����˷�Ӧ���淴Ӧ���ɿ�����ϸ�����ۣ��������۾��кܸߵķ�Ӧ���ԣ��ڿ�������ײ�������ʱ��ȼ�գ������׳ơ�����������д���˷�Ӧ�Ļ�ѧ����ʽ��
Fe3O4+4H2$\frac{\underline{\;����\;}}{\;}$3Fe+4H2O��
��2��שҤ��ճ��������ש����ɫ���ڵ�ש��ʱ��Ҥ���¶ȿɴ�1150�棬Fe2O3��H2��Ӧ����FeO��д���÷�Ӧ�Ļ�ѧ����ʽFe2O3+H2$\frac{\underline{\;1150��\;}}{\;}$2FeO+H2O��
| A�� | NaOH | B�� | MgCl2 | C�� | Na2CO3 | D�� | CaO |
| A�� | ʳ�︯�� | B�� | �̻���ը | C�� | ������ | D�� | ʪ�·����� |