��Ŀ����
2����Ҷ�к��зḻ���л�������ij��ѧ�о�С��ⶨ��Ҷ�и�Ԫ�غ�����ʵ�鷽�����£�����֪��Ҷ��������Ԫ�ضԸ����ӵIJⶨ��Ӱ�죬����ҪԤ�ȳ�ȥ����Һ�������ӡ���������ȫת��Ϊ�������������pH��6���ң����������ڳ����۳����ٳ�ȡ100g����IJ�Ҷ���������ʹ��Ҷ�һ��������в�ĥϸ�������ձ��У�Ȼ�����200mL���ᣬ���衢���ˡ�ϴ�ӣ�
�������������Һ����μ���ϡ����������Һ��������Һ��pHΪ6��7���ң��ټ������30min�����˺�õ���Һ�ͳ�����
����������õ���Һ�м���������ˮ̼���ƣ���ֽ��裬����ַ�Ӧ���ˡ�ϴ�ӣ��õ���Һ�ͳ�������ش��������⣺
��1����Ҷ�зḻ���л���������ֲ������˹�����ã�����д�����ֱ���ʽ��������̼+ˮ$��_{Ҷ����}^{��}$�л������������+����
��2������������ջһ���Ŀ����ȥ����Ҷ�е��л��
��3��������м������30min��Ŀ����ʹ������������������������ڹ��ˣ�
��4�������Ҷ�и�Ԫ�صĺ�������Ҫ�ⶨ�������Dz���������ɳ�����������
���� ��1����ɫֲ��ͨ��Ҷ�������ù��ܰѶ�����̼��ˮ�ϳ��л��������������ͬʱ�ͷų������Ĺ��̽й�����ã�
��2������һ���Ϊ������л���������ˮ�����Σ��л���������ࣨ��Ҫ�ǵ��ۣ���֬���������ʵȣ��л����ܹ�ȼ���ͷų����������о�������ת��Ϊ�����������е�ˮ������ȼ�յĹ����У���������ˮ������ʣ��Ļҽ��������Σ�
��� �⣺��1����ɫֲ��ͨ��Ҷ�������ù��ܰѶ�����̼��ˮ�ϳ��л��������������ͬʱ�ͷų������Ĺ��̽й�����ã���br/���䷴Ӧʽ�ɱ�ʾΪ��������̼+ˮ$��_{Ҷ����}^{��}$�л������������+������
��2������������ջһ���Ŀ���� ȥ����Ҷ�е��л��
��3��������м������30min��Ŀ���� ʹ������������������������ڹ��ˣ�
��4��������м���������ˮ̼���ƣ�̼��������ӷ�����ѧ��Ӧ�����ɳ��������ݳ��������Ϳ��Լ������Ҷ�и�Ԫ�صĺ�����������Ҫ�ⶨ�������Dz���������ɳ�����������
�ʴ�Ϊ����1��������̼+ˮ$��_{Ҷ����}^{��}$�л������������+������
��2��ȥ����Ҷ�е��л����������
��3��ʹ������������������������ڹ��ˣ�
��4������������ɳ�����������
���� ���������ۺ����ÿ�ѧ֪ʶ���������������ǽ���Ĺؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д���1�����ȼ���Ӵ��������Żᷢ�ȣ�ԭ������Ҫ��������ˮ��ͬ���òŻ����⣮
��2����ȡůƬ���е��Ȼ��������Ǣڣ�����ţ�
������ˮ���� �ڼӿ������� ��û���κ���;
��3����ѧ��ȤС��ļס��Ҷ�λͬѧ�ԡ�ȡůƬ���ijɷֲ�������Ȥ��
��ͬѧ�ⶨ�µġ�ȡůƬ�������۵ĺ�����ȡ5.7g��ȡůƬ����Ʒ��Ͷ��װ��10.0gϡ���ᣨ���������ձ����ձ�����10.0g���У��ڻ�ѧ��Ӧ�����ж��ձ���������γ��������ݼ�¼���±���
| ��Ӧʱ�� | t0 | t1 | t2 | t3 | t4 |
| �ձ���ҩƷ����/g | 25.7 | 25.6 | 25.5 | 25.5 | m |
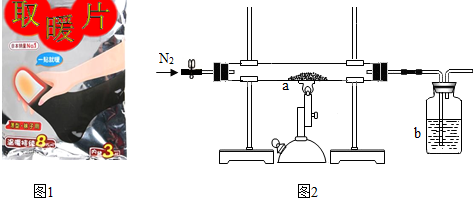
����ͬѧ�ⶨһ���ѱ��ʡ�ȡůƬ����Fe2O3������������ȡ10g�ѱ��ʡ�ȡůƬ����Ʒ�������ľ̿�ۻ�ϣ���ͼ2��ʾװ�����ʵ�飮
��ʵ���м���ǰҪ����ͨ��һ��ʱ�䵪�����������ž�װ���ڵĿ�����ֹͣ���Ⱥ�Ҫ����ͨ��һ��ʱ�䵪��������ᵼ�²ⶨ�Ľ��ƫС���ƫ����ƫС������
�ڳ�ַ�Ӧ����ͬѧ�ⶨb�е��Լ�������3.3g��b�е��Լ�������������Һ��������Ӧ�Ļ�ѧ����ʽΪCO2+2NaOH�TNa2CO3+H2O��
�������ͬѧ����ѱ��ʵġ�ȡůƬ����Ʒ��Fe2O3������������
| A�� | �٢ڢ� | B�� | �٢ڢ� | C�� | �ڢۢ� | D�� | �٢ۢ� |
| ͬѧ | ��Һ�м���������� |
| �� | Na2SO4��Na2CO3 |
| �� | Na2SO4��BaCl2��Na2CO3 |
| �� | Na2SO4��NaCl��Na2CO3 |
| A�� | ����BaCl2��Na2SO4��Na2CO3�������棬����С��Ľ���һ���Dz���ȷ�� | |
| B�� | С������Һ�м�����������ϡ���ᣬ��Ŀ���Ǽ���CO32-�Ĵ��ڲ������ȥ | |
| C�� | С������������Һ�м�����������Ba��NO3��2��Һ�����������Ƿ����SO42-�����������ij�����ȫ���� | |
| D�� | С��ͬѧ�ڱ�����Һ�м�����������BaCl2��Һ�����˺�����Һ�м�����������Һ�����а�ɫ������˵��ԭ��Һ�к�Cl-����С���Ľ���Ҳ�Ǵ���� |

