��Ŀ����
�������ڽ�����������ʱ��������Ҫ�����ã�
��1��5%�İ״���ɱ������ù�����������ã��״��е��ܼ��� ��������Ϊ���ᣨCH3COOH������120g�ð״ף�����̼Ԫ�ص������� ��
��2������ˮ�����õ��������ж������ȣ�ClO2������ȡClO2��Ӧ�Ļ�ѧ����ʽ���£�2NaClO3+4HCl�T2ClO2��+Cl2��+2X+2NaCl������X�Ļ�ѧʽΪ�� ��NaClO3��ClԪ�صĻ��ϼ�Ϊ �ۣ�
��3����ҵ�ϲ���������������ȡClO2�����к�����2.5%��NaClO3����213�֣������Ͽ��Եõ�ClO2�������Ƕ��٣�
��1��5%�İ״���ɱ������ù�����������ã��״��е��ܼ���
��2������ˮ�����õ��������ж������ȣ�ClO2������ȡClO2��Ӧ�Ļ�ѧ����ʽ���£�2NaClO3+4HCl�T2ClO2��+Cl2��+2X+2NaCl������X�Ļ�ѧʽΪ��
��3����ҵ�ϲ���������������ȡClO2�����к�����2.5%��NaClO3����213�֣������Ͽ��Եõ�ClO2�������Ƕ��٣�
���㣺��Һ�����ʺ��ܼ������ϵ���ж�,�й�Ԫ�ػ��ϼ۵ļ���,��������ijԪ�ص���������,�����غ㶨�ɼ���Ӧ��,���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺��ѧʽ�ļ���,�йػ�ѧ����ʽ�ļ���,��ѧ����������غ㶨��,��Һ����Һ���ܽ��
��������1���״��Ǵ����ˮ��Һ����������ijԪ�ص�����=�û��������������Ԫ�ص�����������
��2�����ݻ�ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬�ɷ�Ӧ�Ļ�ѧ����ʽ2NaClO3+4HCl�T2ClO2��+Cl2��+2X+2NaCl���ƶϷ�Ӧ��������X�ķ��ӹ��ɣ�Ȼ��ȷ��X���ʵĻ�ѧʽ�����ݻ�ѧʽ�������ij��Ԫ�صĻ��ϼۣ�
��3�����ݷ���ʽ���㣮
��2�����ݻ�ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬�ɷ�Ӧ�Ļ�ѧ����ʽ2NaClO3+4HCl�T2ClO2��+Cl2��+2X+2NaCl���ƶϷ�Ӧ��������X�ķ��ӹ��ɣ�Ȼ��ȷ��X���ʵĻ�ѧʽ�����ݻ�ѧʽ�������ij��Ԫ�صĻ��ϼۣ�
��3�����ݷ���ʽ���㣮
����⣺��1���״��Ǵ����ˮ��Һ�������ܼ�Ϊ��ˮ����120g�ð״ף�����̼Ԫ�ص�����Ϊ120g��5%��
��100%=2.4g��
��2���ɷ�Ӧ�Ļ�ѧ����ʽ2NaClO3+4HCl�T2ClO2��+Cl2��+2X+2NaCl����֪��
��Ӧǰ ��Ӧ��
Naԭ�� 2 2
Clԭ�� 6 6
Oԭ�� 6 4
Hԭ�� 4 0
���ݻ�ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬������X��2�������к���2��Oԭ�Ӻ�4��Hԭ�ӣ���ÿ��X������2��Hԭ�Ӻ�1��Oԭ�ӹ��ɣ�������X�Ļ�ѧʽΪH2O����Ϊ��Ԫ�صĻ��ϼ���-2�ۣ���Ԫ�صĻ��ϼ���+1�ۣ�����Ԫ�صĻ��ϼ�Ϊx��+1+x+��-2����3=0�����x=+5�ۣ�
��3���������Ͽ��Եõ�ClO2������Ϊy��
2NaClO3+4HCl�T2ClO2��+Cl2��+2H2O+2NaCl
213 135
213t��97.5% y
=
y=131.625t
�ʴ�Ϊ����14�֣�
��1��ˮ�� 2.4��
��2����ÿ��2�֣�
��3��131.625t��6�֣�
| 12��2 |
| 12+3+12+32+1 |
��2���ɷ�Ӧ�Ļ�ѧ����ʽ2NaClO3+4HCl�T2ClO2��+Cl2��+2X+2NaCl����֪��
��Ӧǰ ��Ӧ��
Naԭ�� 2 2
Clԭ�� 6 6
Oԭ�� 6 4
Hԭ�� 4 0
���ݻ�ѧ�仯ǰ��ԭ�ӵ����ࡢ��Ŀ���䣬������X��2�������к���2��Oԭ�Ӻ�4��Hԭ�ӣ���ÿ��X������2��Hԭ�Ӻ�1��Oԭ�ӹ��ɣ�������X�Ļ�ѧʽΪH2O����Ϊ��Ԫ�صĻ��ϼ���-2�ۣ���Ԫ�صĻ��ϼ���+1�ۣ�����Ԫ�صĻ��ϼ�Ϊx��+1+x+��-2����3=0�����x=+5�ۣ�
��3���������Ͽ��Եõ�ClO2������Ϊy��
2NaClO3+4HCl�T2ClO2��+Cl2��+2H2O+2NaCl
213 135
213t��97.5% y
| 213 |
| 213t��97.5% |
| 135 |
| y |
y=131.625t
�ʴ�Ϊ����14�֣�
��1��ˮ�� 2.4��
��2����ÿ��2�֣�
��3��131.625t��6�֣�
���������⿼������Һ��ɡ������غ㶨�ɺͷ���ʽ����ȣ���ɴ��⣬�����������е�֪ʶ�������ṩ����Ϣ���У�

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
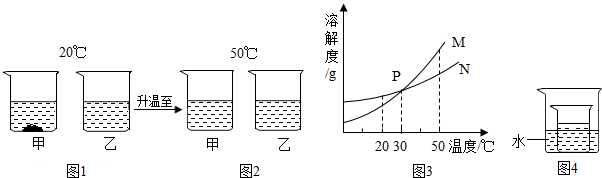
��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ������й�������ȷ���� ��������

| A��20��ʱ�������ʵı�����Һ����������������40% |
| B��30��ʱ��Ҫʹ������Ϊ60�˵ļ����ܽ⣬��ˮ��������� |
| C��10��ʱ���ס����������ʱ�����Һ����������� |
| D����ȥ�����������������ʿɲ�ȡ�����ķ��� |
�����й�ˮ��˵������ȷ���ǣ�������
| A��ˮ��ά�����������ͽ�����Ӫ����֮һ |
| B��Ӳˮ����ˮ�����÷���ˮ���Լ��� |
| C��ˮͨ���ֽܷ⣬˵��ˮ����������������� |
| D��������ͨ�����ˮ���Խ���ˮ��Ӳ�� |
 ��ѧ���������ǻ�ѧ����˼��ͷ�����ͷ���е��ۺ��������������ǻ�ѧѧϰ�����ս���ͶԻ�ѧ��ʶ����߾��磮 ��ˮ��֪ʶΪ������������Ļ�ѧ�ۣ���ش��������⣺
��ѧ���������ǻ�ѧ����˼��ͷ�����ͷ���е��ۺ��������������ǻ�ѧѧϰ�����ս���ͶԻ�ѧ��ʶ����߾��磮 ��ˮ��֪ʶΪ������������Ļ�ѧ�ۣ���ش��������⣺