��Ŀ����
17����������Ҫ�ɷ��Ƕ���������FeS2������һ����Ҫ�Ļ���ԭ�ϣ��������Ʊ�����������Ȼ������乤ҵ����ʾ��ͼ��ͼ�������ϡ�Fe+2FeCl3=3FeCl2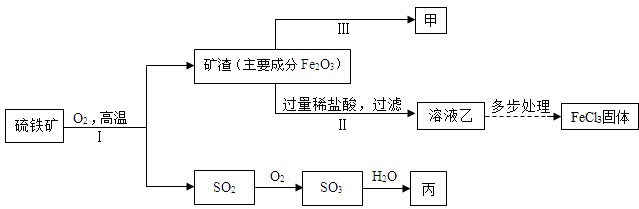
��1�����з�Ӧ�Ļ�ѧ����ʽ��Fe2O3+6HCl=2FeCl3+3H2O��
���з�Ӧ�Ļ�ѧ����ʽ��3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��
��2������������̣����漰�ĺ���������У���Ԫ�صĻ��ϼ���-1��+6��+4����ͼ���ֵĺ���Ԫ�ص������У��������������SO3��SO2���ѧʽ����
��3��������Һ�Һͱ���ϡ��Һ�зֱ���������ļף��۲쵽������ͬ���Dz���ͬ��������Һ�ɻƱ�dz�̣����������ݲ�����
��4������ת������������ʧ��150t��FeS280%���������������Ƶ����������Ϊ196�֣�
���� ��1�������������������ᷴӦ�����Ȼ�����ˮ�Լ���������һ����̼��Ӧ�������Ͷ�����̼�Ĺ�����д����ʽ���ɣ�
��2�����ݹ������漰������Ļ��������������Ļ��ϼۣ�
��3�����ݷ�Ӧ�ľ�������������
��4�����ݻ�ѧ����ʽ���йؼ���������
��� �⣺��1��II��������������ķ�Ӧ������ʽΪ��Fe2O3+6HCl=2FeCl3+3H2O�����ڸù��������������Ȼ���������Ĺ��̣���ͼʾ������֪��Ӧ���ǽ�����������III�ķ�Ӧ�����ǣ�3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��
��2�������������漰�������к�������ʷֱ���FeS2��SO3��SO2���ڶ�����������Ԫ����+2�ۣ������ڻ��������������ϼ۴�����Ϊ�㣬�����Ԫ�ػ��ϼ���-1�ۣ���SO3����Ԫ����-2�ۣ�������Ԫ����+6�ۣ���SO2����Ԫ����-2�ۣ�������Ԫ����+4�ۣ����Ի��ϼ۷ֱ���-1��+6��+4���������������SO3��SO2
��3����ͼʾ��֪�ҵ���Һ���Ȼ���������������Һ�����ķ���ᵼ�·���Fe+2FeCl3=3FeCl2�������û��������е���Ӷ����������Ĺ��̣�������������ͬ��
��4������ת������������ʧ������Ԫ�ص�������ͬ����150t��FeS280%���������������Ƶ������������x��
FeS2��2H2SO4
120 196
150t��80% x
���ݣ�$\frac{120}{196}=\frac{150t��80%}{x}$���x=196t
�ʴ�Ϊ��1��Fe2O3+6HCl=2FeCl3+3H2O��3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2��
��2��-1��+4��+6��SO2SO3
��3������ͬ��������Һ�ɻƱ�dz�̣����������ݲ�����
��4��196t��
���� �����Ƕ������й�֪ʶ�Ŀ��飬����Ĺؼ��Ƕ�����ұ���������Լ���ѧ�������������գ�

 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�| ʵ����� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ����ϡ������������ˣ� | 20 | 20 | 20 | 20 |
| ��ַ�Ӧ��ʣ�������������ˣ� | 17.2 | 14.4 | 12.0 | m |
��1������������m��ֵΪ12.0��
��2������Ʒ��������������Ϊ40%��
��3������ϡ��������������������Ƕ��٣�
| A�� | �����������л������� | B�� | ����������Ԫ������������С | ||
| C�� | ��������8��Ԫ����� | D�� | �����ʵ�һ�������к���77��ԭ�� |
 �ס��ҡ����������ʵ��ܽ��������ͼ��ʾ������������ȷ���ǣ�������
�ס��ҡ����������ʵ��ܽ��������ͼ��ʾ������������ȷ���ǣ�������| A�� | �ҵ��ܽ����� | |
| B�� | һ��������Һ��60�潵����40��ʱ���������ľ������ | |
| C�� | 40��ʱ���Һͱ��ı�����Һ�����ʵ�����������ͬ | |
| D�� | 40��ʱ��100gˮ�м���50g����������Һ�����ʵ���������Ϊ33.3% |





