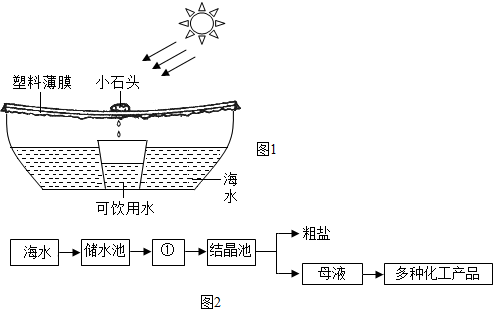
��Ŀ����
2����ѧ֪ʶ�����ǵ��ճ���������������еĹ�ϵ����1�������˵�θ����HCl��д��ѧʽ�������θҺ���ڹ��������θ�������ú�������������ҩ��ɻ��ⲡʹ�������ƵĻ�ѧԭ��Ϊ���û�ѧ����ʽ��ʾ��3HCl+Al��OH��3=AlCl3+3H2O��
��2�����ӡ�ÿ���������桷�ԡ��Ƿ�������Ƥ���½�����ҩ�ý��ҡ��ع⣬���ƳɵĽ��������ؽ��������꣬�ؽ����Ķ�����Ҫ����������������ø�ĩ�SH����϶���������ʳ�ؽ����κɾ��������ţ�̣������ʣ��ⶾ��һ�����ʵ����ƣ���
���� ��1���������������������������кͷ�Ӧ�������ɣ�
��2�������ؽ������ж���ԭ���жϣ����ƻ�����ĵ����ʽṹ��ʹ֮ʧȥ�������ܣ�
��� �⣺��1��θ���к������ᣬ��������������������кͷ�Ӧ����Ӧ����ʽΪ��3HCl+Al��OH��3=AlCl3+3H2O
��2���ؽ������ж���ԭ�����ƻ�����ĵ����ʽṹ���������Ҫ�ɷ��ǵ����ʣ������塢������ţ���к��е����ʣ����ü����塢������ţ�̣��ɷ�ֹ���屾���ĵ����ʱ��ƻ��������ڽⶾ��
�ʴ�Ϊ����1��HCl��3HCl+Al��OH��3=AlCl3+3H2O
��2��ţ�̣������ʣ���
���� �����ѶȲ��������������������к�θ���ԭ�����ж�ԭ������ȷ��������Ĺؼ����ڣ�

��ϰ��ϵ�д�
�����Ŀ
10������ͷ�����ŷ����Ȼ���ᣬҪ��ȥ��ζ�����������м��루������
| A�� | �ռ� | B�� | ��ʯ�� | C�� | ���� | D�� | ʳ�� |
17����ͼ��ʾ���ܷ�װ�ã��ȵ�����ƽƽ�⣬����һ��ʱ�䣬���ֵ�����ǣ�������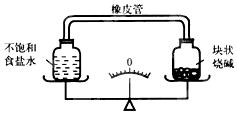

| A�� | ָ��ƫ��ʳ����Һһ����Ũ���ռ���� | |
| B�� | ָ��ƫ�ң�ʳ����Һһ�����ͣ��ռ�� | |
| C�� | ָ��ƫ��ʳ����Һһ����Ũ���ռ�� | |
| D�� | ָ��ƫ�ң�ʳ����Һһ����Ũ���ռ�� |
14����֪FeO��Fe2O3��ɵĻ�����У���������������Ϊ14��5����������FeO��Fe2O3�������ʵ������ȿ����ǣ�������
| A�� | 1��8 | B�� | 2��1 | C�� | 4��5 | D�� | 9��10 |
12������ݱ������ʻش������й�����
��1���ϱ��е������������������Fe2O3���û�ѧʽ��ʾ����
��2��������Ƥ����һ�ּ������������أ�Ƥ��մ�����������̣�����Ϊ��ѡ�����Т�
��ͿĨֹ�������������д��
�ٴ��� ����ʯ�� �۴��
| ��� | �� | �� | �� | �� | �� |
| ���� | ���� | ������ | ��ʯ�� | ���� | ���� |
��2��������Ƥ����һ�ּ������������أ�Ƥ��մ�����������̣�����Ϊ��ѡ�����Т�
��ͿĨֹ�������������д��
�ٴ��� ����ʯ�� �۴��