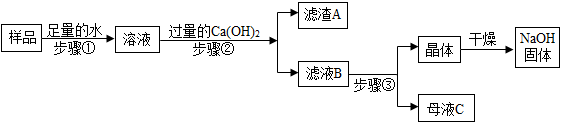
��Ŀ����
3�� Ϊ�ⶨij������ͭ��п��Ʒ��п������������ȡ��Ʒ20��װ��һ��������ȡϡ����200�ˣ�ƽ���ֳ�4�ݲ���4�μ��������У��ⶨ�����������������������±���
Ϊ�ⶨij������ͭ��п��Ʒ��п������������ȡ��Ʒ20��װ��һ��������ȡϡ����200�ˣ�ƽ���ֳ�4�ݲ���4�μ��������У��ⶨ�����������������������±���| ϡ��������� | ��һ��50g | �ڶ���50g | ������50g | ���Ĵ�50g |
| �������������� | 0.2g | m | 0.6g | 0.6g |
��2��20����Ʒ��ϡ���ᷴӦ������������0.6g��
��3��������Ʒ��п�����������Ƕ��٣���д��������̣�
��4������������������������ϡ����Ĺ�ϵ����ͼ��
���� ��1�����ݵ�һ�μ���50gϡ������������������Ϊ0.2g�������μ���50gϡ������������������Ϊ0.6g��˵��ÿ����50gϡ������������������Ϊ0.2g��
��2�����ݱ������ݷ�����
��3������������������������ϻ�ѧ����ʽ���㣮
��� �⣺��1�����ݵ�һ�μ���50gϡ������������������Ϊ0.2g�������μ���50gϡ��������������������Ϊ0.6g��˵��ÿ����50gϡ������������������Ϊ0.2g���ڶ�������50gϡ������������������Ϊ0.2g��������������������Ϊ0.4g�����0.4g��
��2�����ݱ������ݵ��Ĵμ���50gϡ����������������������Ϊ0.6g�����Դ�ʱ��Ӧ�Ѿ�ֹͣ��������������0.6g�����0.6��
��3������Ʒ��п������Ϊx
Zn+H2SO4�TZnSO4+H2��
65 2
x 0.6g
$\frac{65}{x}=\frac{2}{0.6g}$
x=19.5g
��Ʒ��п������������$\frac{19.5g}{20g}��100%$=97.5%
����Ʒ��п������������97.5%
��3������ÿ����50gϡ������������������Ϊ0.2g��˵��������ǡ����ȫ��Ӧ��������������������ϡ����Ĺ�ϵ����ͼΪ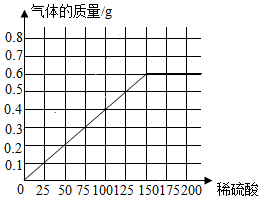 ��
��
���� ������Ҫ����ѧ������ȫ��Ӧ�ĸ������ʶ���Լ����û�ѧ����ʽ������������ʽ���м����������

| A�� | ���� | B�� | �ӿ� | C�� | �ı� | D�� | ���� |
[�������1]������������Һ�Ƿ�������أ�
[ʵ��̽��1]
| ʵ����� | ʵ������ | ʵ����� |
| ȡ��������Һ���Թ��У�����Һ�еμ�ϡ���ᣬ�������� | ������ð���� | ����������Һһ�������ˣ� |
[���������]
����1������������Һ���ֱ��ʣ�
����2������������Һȫ�����ʣ�
[��������]��1���Ȼ�����Һ�����ԣ�
��2���Ȼ�����Һ����̼������Һ��Ӧ��CaCl2+Na2CO3=CaCO3��+2NaCl
[ʵ��̽��2]
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��1��ȡ��������Һ���Թ��У�����Һ�еμӹ������Ȼ�����Һ���������� | �����ɣ� | ˵��ԭ��Һ��һ����̼���ƣ� |
| ��2��ȡ���裨1���Թ��е������ϲ���Һ���μӷ�̪��Һ�� | ��Һ���ɫ�� | ˵��ԭ��Һ��һ���У� |
[��˼������]��1������������Һ¶���ڿ��������ױ��ʣ���д����ط�Ӧ�Ļ�ѧ����ʽ��CO2+2NaOH=Na2CO3+H2O������[ʵ��̽��2]�У�С�������������������Һ�����Ȼ�����Һ������Ϊ�÷��������У�����С������С�����
[������Ӧ��]����������Һ���ױ��ʣ������ܷⱣ�森ʵ���ұ����ܷⱣ���ҩƷ���кܶ࣬������һ����Ũ����ӷ��Ե�ҩƷ��Ũ���ᡢ��ˮ�ȣ�������ˮ�Ե�ҩƷ��Ũ���ᡢ��ʯ�ҡ������ʯ�ң�
��3��ȡ�������ֱ��ʵ�����������Һ100g�������м�������������������Һ����ȫ��Ӧ��õ�1.97g��ɫ����������������������Һ��̼���Ƶ�������������д��������̣�
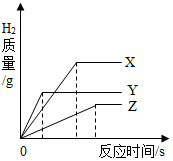 ����ͬ�������ͬ��������������ϡ���ᣬ�ֱ�ε���������������С��ͬ��X��Y��Z���ֽϻ��ý����У�����H2�������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������
����ͬ�������ͬ��������������ϡ���ᣬ�ֱ�ε���������������С��ͬ��X��Y��Z���ֽϻ��ý����У�����H2�������뷴Ӧʱ��Ĺ�ϵ��ͼ��ʾ������˵������ȷ���ǣ�������| A�� | ���ֽ����Ļ��˳��ΪX��Z��Y | |
| B�� | ��������������˳��Ϊ X��Y��Z | |
| C�� | ��Ӧ���ʱ����ϡ���������˳��ΪX��Y��Z | |
| D�� | ���ԭ�������ɴ�С��˳��Ϊ X��Y��Z |
| A�� | �ɱ��˹����� | B�� | ���ͨ�緢�� | C�� | Һ̬�ⷢ���� | D�� | ˮ��� |

 ��ͼ�Ǽס��ҡ������ֹ������ʵ��ܽ������ͼ������ͼʾ�ش��������⣺
��ͼ�Ǽס��ҡ������ֹ������ʵ��ܽ������ͼ������ͼʾ�ش��������⣺ ��ʦ����ͼ��ʾװ��Ϊͬѧ��������ʵ�飺Aװ�ü���ƿ��װ�������ԼΪ1��1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᣮ
��ʦ����ͼ��ʾװ��Ϊͬѧ��������ʵ�飺Aװ�ü���ƿ��װ�������ԼΪ1��1�ĵ���������a�Ļ�����壬ע������װ����������ɫ��Һb��Bװ����ʢ��������ɫʯ����Һ��Cװ����ʢ�����������������Dװ����ʢ��������ϡ���ᣮ