��Ŀ����
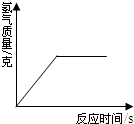
10�� ijѧУ��ѧ��ȤС��Ϊ��̽��ʵ�����о��õ�NaOH�ı��ʳ̶ȣ�������ͼ��
ijѧУ��ѧ��ȤС��Ϊ��̽��ʵ�����о��õ�NaOH�ı��ʳ̶ȣ�������ͼ�����о��������ȳ�ȡ13.3g ��NaOH��Ʒ������ΪNa2CO3���������Һ��Ȼ������Һ����μ�����������Ϊ14.6%��ϡ���ᣬ��������CO2�������ⶨNa2CO3���������Ӷ���һ��ȷ����Ʒ��NaOH�ı��ʳ̶ȣ������ʳ̶���ָ�ѱ��ʵ�NaOH��ԭNaOH �е�����������
��1��ʵ���ü���ϡ��������������CO2�����������ϵ��ͼ��ʾ������Ʒ��Na2CO3�������Ƕ��٣�
��2����ʵ���������NaOH��Ӧ���õ��������ʵ�������
��3������Ʒ��NaOH�ı��ʳ̶ȣ�
���� ��1���������������ܺͿ����еĶ�����̼��Ӧ����̼���ƺ�ˮ���ܺ�ϡ���ᷴӦ�����Ȼ��ƺ�ˮ��̼���ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ö�����̼���������̼���Ƶ��������ɣ�
��2�������������Ƶ��������ʵ���������NaOH��Ӧ���õ��������ʵ��������ɣ�
��3������̼���Ƶ�����������ʵ��������Ƶ����������������Ʒ��NaOH�ı��ʳ̶ȼ��ɣ�
��� �⣺��1����̼���Ƶ�����Ϊx��
��ͼ����Ϣ��֪������������̼��������2.2g��
Na2CO3+2HCl�T2NaCl+H2O+CO2����
106 44
x 2.2g
$\frac{106}{x}=\frac{44}{2.2g}$
x=5.3g
��2������������Ʒ�Ӧ���Ȼ�������Ϊy��
���Ȼ��ⷴӦ��������������Ϊ��13.3g-5.3g=8g��
NaOH+HCl=NaCl+H2O��
40 36.5
8g y
$\frac{40}{8g}=\frac{36.5}{y}$
y=7.3g��
��3������ʵ�������������Ϊz��
2NaOH+CO2�TNa2CO3+H2O��
80 106
z 5.3g
$\frac{80}{z}=\frac{106}{5.3g}$
z=4g
��NaOH�ı��ʳ̶�Ϊ��$\frac{4g}{13.3g-5.3g+4g}$��100%=33.3%��
�𣺣�1������Ʒ��Na2CO3��������5.3g��
��2��ʵ���������NaOH��Ӧ���õ��������ʵ�����Ϊ7.3g��
��3����NaOH�ı��ʳ̶�Ϊ33.3%��
���� ������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�������ͬʱ�����˷���ͼ�����ݵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

| ѡ�� | A | B | C | D |
| ʵ��Ŀ�� | ��ȥ���������� ������̼���� | ��ϴ����Ʒ ��������� | �����Ȼ��� ��Һ��ϡ���� | ����һ����̼���Ƿ��������������̼ |
| ����1 | ��ˮ�ܽ� | ������ϡ���� | ��п�� | ��ȼ |
| ����2 | ��ϡ���� | ��ˮϴ�� | ��ʯ����Һ | ͨ�����ʯ��ˮ |
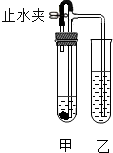 ijͬѧ����ͼ��ʾװ�ý���ʵ�飨ͼ������̨������������ȥ�����ڼ��Թ��м����Լ���������Ƥ����������ֹˮ�У����Թ���������ð����һ��ʱ���ر�ֹˮ�У����Թ���Һ����������Һ�ɳ������ǣ���������ʵ������ļס����Թ���Ӧ������Լ��ǣ�������
ijͬѧ����ͼ��ʾװ�ý���ʵ�飨ͼ������̨������������ȥ�����ڼ��Թ��м����Լ���������Ƥ����������ֹˮ�У����Թ���������ð����һ��ʱ���ر�ֹˮ�У����Թ���Һ����������Һ�ɳ������ǣ���������ʵ������ļס����Թ���Ӧ������Լ��ǣ�������| A | B | C | D | |
| �� | Zn��ϡH2SO4 | Cu��ϡH2SO4 | CaCO3��ϡHCl | Na2CO3��ϡH2SO4 |
| �� | BaCl2 | Ba��OH��2 | KNO3 | NaCl |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ����ʯ��ˮ | B�� | ��ɫʯ����Һ | C�� | �����ǵ�ľ�� | D�� | ���� |
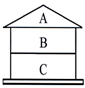 ͬѧ���ԸǷ��ӵķ�ʽ�ܽ�����������ʼ�Ĺ�ϵ��A��B��C��Ϊ��������ϡ������ڵ�����֮����ɷ�����Ӧ���ش��������⣺
ͬѧ���ԸǷ��ӵķ�ʽ�ܽ�����������ʼ�Ĺ�ϵ��A��B��C��Ϊ��������ϡ������ڵ�����֮����ɷ�����Ӧ���ش��������⣺