��Ŀ����
1��ʵ���ҿ����գ�ij��ѧ��ȤС���ͬѧ����ʦ��ָ���£��������ͼ��ʾʵ��װ�ý���������ȡ�����ʵ�̽������ش��й����⣺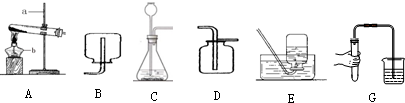
��1����д��ͼ�б�����ĸ���������ƣ�a����̨ b�ƾ��ƣ�
��2��ͨ����Gͼ��ʾ�ķ�������װ�õ������Լ�飬���װ�ò�©�������Կ������ܿ�������ð���������ɿ�һ��ʱ������Բ���ˮ�У������Կ����������ǵ����н���һ��ˮ����
��3��ʵ�����ü��ȸ������ȡ����ʱ��Ӧѡ�õķ���װ����A����дװ�õ���ĸ���ţ���ͬ������Ӧ�Ļ�ѧ����ʽ��2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2������װ�û���һ��ȱ�����Թܿ�û�����ţ���ѡ�ù���������������Ӧѡ�õķ���װ���ǣ�C����Ӧ�Ļ�ѧ����ʽ��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��4�������װ��E�ռ����壬ʵ����Ϻ�Ӧ���Ƴ�����Ȼ����Ϩ��ƾ��ƣ��ռ�����������ѡ��D��˵���������ܶȱȿ������ܶȴ�
���� ����̨�dz��õļг��������ƾ����dz��õļ���������ͨ����Gͼ��ʾ�ķ������װ�õ������ԣ����װ�ò�©�������Կ������ܿ�������ð���������ɿ�һ��ʱ������Բ���ˮ�У������Կ����������ǵ����н���һ��ˮ������ȡװ�ð������ȺͲ���������֣������˫��ˮ�Ͷ��������������Ͳ���Ҫ���ȣ�����ø�����ػ����������������Ҫ���ȣ����ȸ������ʱ���Թܿ�Ҫ��һ��������Ϊ�˷�ֹ������ط�ĩ���뵼�ܣ��������ܶȱȿ������ܶȴ�������ˮ��������������ſ���������ˮ���ռ���ʵ�����Ӧ���Ƴ����ܣ���Ϩ��ƾ��ƣ���ԭ���ǣ���ֹˮ������ʹ�Թ�ը�ѣ�
��� �⣺��1������̨�dz��õļг��������ƾ����dz��õļ����������ʴ�Ϊ������̨���ƾ��ƣ�
��2��ͨ����Gͼ��ʾ�ķ������װ�õ������ԣ����װ�ò�©�������Կ������ܿ�������ð���������ɿ�һ��ʱ������Բ���ˮ�У������Կ����������ǵ����н���һ��ˮ�����ʴ�Ϊ��װ�õ������ԣ������н���һ��ˮ����
��3������ø����������������Ҫ���ȣ����ȸ������ʱ���Թܿ�Ҫ��һ��������Ϊ�˷�ֹ������ط�ĩ���뵼�ܣ�����������ȷֽ���������غͶ������̺�������Ҫע����ƽ�������˫��ˮ�Ͷ��������������Ͳ���Ҫ���ȣ����������ڶ�������������������������ˮ��������Ҫע����ƽ���ʴ�Ϊ��A��2KMnO4$\frac{\underline{\;\;��\;\;}}{\;}$K2MnO4+MnO2+O2�����Թܿ�û�����ţ�C��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��4��ʵ�����Ӧ���Ƴ����ܣ���Ϩ��ƾ��ƣ���ԭ���ǣ���ֹˮ������ʹ�Թ�ը�ѣ��������ܶȱȿ������ܶȴ�������ˮ��������������ſ���������ˮ���ռ����ʴ�Ϊ���Ƴ����ܣ�Ϩ��ƾ��ƣ�D����
���� ��������Ҫ���������������ơ��������ȡװ�ú��ռ�װ�õ�ѡ��ͬʱҲ�����˻�ѧ����ʽ����д��ע������ȣ��ۺ��ԱȽ�ǿ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����йأ����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�

| ��� | ����ϡ�����������g�� | ʣ������������g�� |
| ��1�� | 10 | 5.5 |
| ��2�� | 10 | M |
| ��3�� | 10 | 1.2 |
| ��4�� | 10 | 1.2 |
��2��M��ֵΪ3
��3��ʯ��ʯ��Ʒ��̼��Ƶ���������85%
��4������ʵ���в����Ķ�����̼��������x���ı���ʽΪ$\frac{100}{6.8g}=\frac{44}{x}$
��5�����ڶ���ʵ���õ��Ļ������ˣ�Ҫ���õ�����Һ���������������Ϊ10%����Һ��Ӧ����Һ�м�ˮ32.7g
��6����36.5%��Ũ��������ʵ����ʹ�õ�40gϡ���ᣬ��Ҫ��ˮ��������20g��
 ��֪����������һ�ֲ�����ˮ�İ�ɫ����������ϡ���ᷴӦ��Ҳ�������������������Һ��Ӧ����������������������Һ��Ӧ�Ļ�ѧ����ʽΪ��Al��OH��3+NaOH�TNaAlO2+2H2O�����ɵ�ƫ���ƣ�NaAlO3��������ˮ����Mg��OH��2���岻����NaOH��Һ�����к�AlCl3��MgCl2�Ļ����Һ50g������Һ����μ���������������Ϊ10%��NaOH��Һ�����ɳ������������NaOH��Һ������ϵ��ͼ��ʾ��
��֪����������һ�ֲ�����ˮ�İ�ɫ����������ϡ���ᷴӦ��Ҳ�������������������Һ��Ӧ����������������������Һ��Ӧ�Ļ�ѧ����ʽΪ��Al��OH��3+NaOH�TNaAlO2+2H2O�����ɵ�ƫ���ƣ�NaAlO3��������ˮ����Mg��OH��2���岻����NaOH��Һ�����к�AlCl3��MgCl2�Ļ����Һ50g������Һ����μ���������������Ϊ10%��NaOH��Һ�����ɳ������������NaOH��Һ������ϵ��ͼ��ʾ�� ������һ����ɫ���д̼�����ζ��Һ�壬�н�ǿ�ĸ�ʴ�ԣ�С����֪�������Ƿ�������ԣ���������̽����
������һ����ɫ���д̼�����ζ��Һ�壬�н�ǿ�ĸ�ʴ�ԣ�С����֪�������Ƿ�������ԣ���������̽����

 ijѧУ��ѧ��ȤС��Ϊ��̽��ʵ�����о��õ�NaOH�ı��ʳ̶ȣ�������ͼ��
ijѧУ��ѧ��ȤС��Ϊ��̽��ʵ�����о��õ�NaOH�ı��ʳ̶ȣ�������ͼ��