��Ŀ����
6��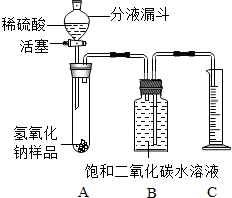 ij��ѧ����С���ͬѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ��������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�
ij��ѧ����С���ͬѧ��Ϊ�˲ⶨʵ������һƿ�治�ƶ����ֱ��ʵ�����������̼���Ƶ��������������������ͼ��ʾ��װ�ã�ͼ������̨�Ѿ���ȥ����ʵ����27�棬101kPa�½��У�ʵ�鲽�����£�
�ٰ�ͼ���Ӻ�װ�ã�
������ƽȷ��ȡ����������Ʒm g������A���Թ��ڣ���B�м���ƿ�ڵ��뱥�Ͷ�����̼ˮ��Һ��ƿ������
�����Һ©���е���ϡ���ᣬ��������ϡ��������Թ������������رջ�������Ӧ��������Ͳ���ռ������Ͷ�����̼ˮ��Һv mL��
�ܼ�������������Ʒ��̼���Ƶ�����������
��ش��������⣺
��1��ȷ�ж��������Ʒ������ʵ�ʵ��������A�������ݲ�����д���������Ʒ������ʵĻ�ѧ����ʽ2NaOH+CO2�TNa2CO3+H2O��
��2����ʵ�鲽��ٺ͢�֮�䣬��ȱ��һʵ�鲽�裬��ʵ�鲽���ǣ����װ�������ԣ�
��3��B�м���ƿʢװ�ı��Ͷ�����̼ˮ��Һ������ˮ���棬��ԭ���ǣ����������̼�ܽ���ˮ�����ģ���ɲⶨ���ƫ�ͣ�
��4���ж�ʵ�鲽����е����ϡ�����ѹ����ı�־�ǵ���ϡ���ᣬA�в��������ݲ�����
��5�����������������Ʋ��ֱ��ʵķ�����
| ʵ�鲽�� | ʵ������ | ʵ����� |
| 1��ȡ��������ˮ���μӹ������Ȼ�����Һ | ������ɫ���� | �������Ʋ��ֱ��� |
| 2�����ã����ϲ���Һ�еμӷ�̪��Һ | ��� |
��7��ȡ10g���ʵ������������ձ��У�����̼Ԫ�ص���������Ϊ6%�����ձ��м���100gһ������������ϡ���ᣨ����������Ӧ�������ձ������ʵ���������107.8g��
���� ��1���������������ʣ��������̼���ƣ���ô������ϡ����ʱ��������壻
��2����ʵ�������������룬���Ա��뱣֤װ�õ����������ã�
��3��������̼������ˮ��
��4�����������Ʊ��ʣ�����������壬�����ٲ�������ʱ��˵����Ӧ��ϣ�
��5���������Ʋ��ֱ��ʣ��ʼ�Ҫ��������̼������Ҫ���������������ƣ�
��6�����������������տ����е�ˮ�ֶ����⣮
��7�����������غ㣬��֪ԭ�����е�̼Ԫ�ص������������ɶ�����̼��̼Ԫ�ص�������
��� �⣺��1�����������ڿ����������տ����е�ˮ�ֶ����⣬Ȼ���������еĶ�����̼��Ӧ�����ʣ������������������ʣ��������̼���ƣ���ѧ����ʽΪ��2NaOH+CO2�TNa2CO3+H2O����ô������ϡ����ʱ�����������̼���壬����A�л�ð�����ݣ�
�ʴ�Ϊ��A�������ݲ�����2NaOH+CO2�TNa2CO3+H2O��
��2����ʵ���������������룬����ʵ��ǰ������װ�õ������ԣ�
�ʴ�Ϊ�����װ��������
��3�������ʵ��Ŀ����ͨ���������̼�����������ҩƷ��̼���Ƶĺ��������Ա��뱣֤������̼��ȷ�ԣ���Ϊ������̼��������ˮ�������ñ��͵Ķ�����̼ˮ��Һ�����Ա��������̼�ļ��٣�
�ʴ�Ϊ�����������̼�ܽ���ˮ�����ģ���ɲⶨ���ƫ�ͣ�
��4�����������Ʊ��ʣ�������ϡ����ʱ����������壬�����ٲ�������ʱ��˵����Ӧ��ϣ�
�ʴ�Ϊ������ϡ���ᣬA�в��������ݲ�����
��5�������������Ʋ��ֱ��ʣ���Ҫ��������̼������Ҫ���������������ƣ�����̼���ƾ��Ǽ���̼������ӣ���ͨ�����Ȼ��Ʒ�Ӧ����̼����������������������ƾ��Ǽ������������ӣ���ͨ������ʹ��ɫ��̪��Һ��������м�����
�ʴ�Ϊ��
| ʵ�鲽�� | ʵ������ | ʵ����� |
| 1��ȡ��������ˮ���μӹ������Ȼ�����Һ | ������ɫ���� | �������Ʋ��ֱ��� |
| 2�����ã����ϲ���Һ�еμӷ�̪��Һ | ��� |
�ʱ����Ϊ������������Ʒ�г���̼���ƣ�����ˮ��
��7�����������غ㣬��֪ԭ�����е�̼Ԫ�ص������������ɶ�����̼��̼Ԫ�ص�������10g���ʵ�����������̼Ԫ�ص�����Ϊ10g��6%=0.6g��������ɵö�����̼������Ϊx
x��$\frac{12}{12+16��2}$��100%=0.6g�����x=2.2g
���Է�Ӧ�������ձ������ʵ�������Ϊ10g+100g-2.2g=107.8g
�𣺷�Ӧ�������ձ������ʵ�������Ϊ107.8g��
���� ���������������ơ�̼���Ƶ����ʣ�֪����������¶���ڿ������ױ��ʣ�������飬��ס��ѧ����ʽ��Na2CO3 +2HCl�T2NaCl+H2O+CO2�����������ĸ��ݻ�ѧ����ʽ���м��㣮

| �¶�/�� | 0 | 20 | 40 | 60 | 80 | |
| �ܽ��/g | NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 |
| KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 | |
| A�� | ��60��ʱKNO3�ı�����Һ������20�棬���ʡ��ܼ�������������� | |
| B�� | 40��ʱ����50g NaCl��50g KNO3�ֱ����100gˮ�У�������Һ������������������� | |
| C�� | ��80��ʱNaCl��KNO3���ֱ�����Һ������20�棬�������������һ����KNO3��NaCl | |
| D�� | ���ϱ����ݻ��Ƴ�NaCl��KNO3���ܽ�����ߣ��������߽����Ӧ���¶ȷ�Χ��0��20�� |
| A�� | ��ˮ | B�� | ��ˮ | C�� | ��ˮ | D�� | ��ˮ |
| A�� | ���ס���Ϊ���ʣ���÷�Ӧһ�����û���Ӧ | |
| B�� | ����Ϊ���壬��ס�����һ���е��� | |
| C�� | ����Ϊ��������ס�����һ����һ�������Ǽ� | |
| D�� | ����Ϊˮ����÷�Ӧһ���Ǹ��ֽⷴӦ |
| A�� | �ϱ����������ڻ� | B�� | ������ɳʯǨ�� | ||
| C�� | ����ʯ�͵��γ� | D�� | ��������ת�� |
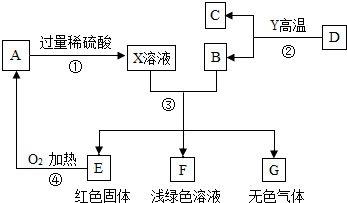 ���� A��B��C��D��E��F��GΪ���������ʣ�����B��E��G���ڵ��ʣ���Ӧ����������ҵ�е���Ҫ��Ӧ������֮����ת����ϵ��ͼ��ʾ��
���� A��B��C��D��E��F��GΪ���������ʣ�����B��E��G���ڵ��ʣ���Ӧ����������ҵ�е���Ҫ��Ӧ������֮����ת����ϵ��ͼ��ʾ��