��Ŀ����
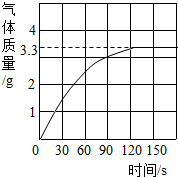 ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ���У�����CO2����������ͼ��ʾ����ʾ����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�� Mg��OH��2+2HCl�TMgCl2+2H2O
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ���У�����CO2����������ͼ��ʾ����ʾ����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�� Mg��OH��2+2HCl�TMgCl2+2H2O��1����ͼ�п��Կ�����12.5gˮ�������ᷴӦ�����ɵĶ�����̼�����
��2��ˮ����̼��Ƶ����������Ƕ��٣�
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1�����ݷ�Ӧ������������ͼ������Ӧ��120sʱ�ų�������̼��������ֵ��Ϊ3.3g��
��2���ų�������̼�����ֵʱ��ˮ����̼�����ȫ��Ӧ�����ݷ�Ӧ�Ļ�ѧ����ʽ���ɷų�������̼�������������Ʒ��̼���������̼���������ˮ����Ʒ�����ȿɼ���ˮ����̼��Ƶ�����������
��2���ų�������̼�����ֵʱ��ˮ����̼�����ȫ��Ӧ�����ݷ�Ӧ�Ļ�ѧ����ʽ���ɷų�������̼�������������Ʒ��̼���������̼���������ˮ����Ʒ�����ȿɼ���ˮ����̼��Ƶ�����������
����⣺��1���ɷ�Ӧ������������ͼ����֪12.5gˮ�������ᷴӦ�����ɵĶ�����̼�����3.3g��
�ʴ�Ϊ��3.3��
��2����12.5gˮ����̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.3g
=
x=7.5g
ˮ����̼��Ƶ���������Ϊ
��100%=60%
��ˮ����̼��Ƶ�����������60%��
�ʴ�Ϊ��3.3��
��2����12.5gˮ����̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.3g
| 100 |
| x |
| 44 |
| 3.3g |
x=7.5g
ˮ����̼��Ƶ���������Ϊ
| 7.5g |
| 12.5g |
��ˮ����̼��Ƶ�����������60%��
����������CO2�������������۵������ʱ�ų����������̼�����ֵ����ˮ����̼�����ȫ��Ӧ��

��ϰ��ϵ�д�
�����Ŀ
��������������������ǣ�������
| A������ | B������ |
| C��һ����̼ | D������� |
 ��ͼΪ�ⶨ������ɵ�ʵ��װ�ã�����ͼʾ�ش��������⣺
��ͼΪ�ⶨ������ɵ�ʵ��װ�ã�����ͼʾ�ش��������⣺ ��ͼ��ʾ���ձ���ʢ��ˮ����ֻ�����ͬ�ȵļ���ƿ�ж������˿������н�ֹˮ�У���Ѹ�ٽ��ֱ�ʢ�й����ס����ȼ�ճ�ȼ��Ѹ������ס�����ƿ�ڣ���ȼ�յIJ���Ϊ��������������ˮ��������ƿ������ȼ����ϣ���ȴ�����º���ֹˮ�У���ɼ�ˮ�ܿ����
��ͼ��ʾ���ձ���ʢ��ˮ����ֻ�����ͬ�ȵļ���ƿ�ж������˿������н�ֹˮ�У���Ѹ�ٽ��ֱ�ʢ�й����ס����ȼ�ճ�ȼ��Ѹ������ס�����ƿ�ڣ���ȼ�յIJ���Ϊ��������������ˮ��������ƿ������ȼ����ϣ���ȴ�����º���ֹˮ�У���ɼ�ˮ�ܿ���� ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ� D��̼�����ԭ������Ϊ12.01
D��̼�����ԭ������Ϊ12.01
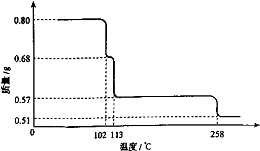 0.80g CuSO4?5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ��
0.80g CuSO4?5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ��