��Ŀ����
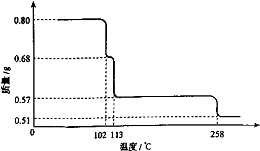 0.80g CuSO4?5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ��
0.80g CuSO4?5H2O��Ʒ������ˮ���̵��������ߣ���Ʒ�������¶ȱ仯�����ߣ���ͼ��ʾ����ش��������⣺
��1����ȷ��200��ʱ�������ʵĻ�ѧʽ
��2��ȡ270��������Ʒ����570�����յõ�����Ҫ�����Ǻ�ɫ��ĩ��һ�����������壬�÷�Ӧ�Ļ�ѧ����ʽΪ
���㣺�йػ�ѧʽ�ļ�����ƶ�,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ
ר�⣺��ѧʽ�ļ���
��������1����ͼ������֪��CuSO4?5H2O���ȵ�102��ʱ��ʼ��ˮ�ֽ⣬113��ʱ�ɵõ����ȶ���һ���м����258��ʱ�Ż�����ֽ⣮��200��ʱʧȥ��ˮ������Ϊ0.80g-0.57g=0.23g��������Ӧ�Ļ�ѧ����ʽ����ȷ����ʱ�������ʵĻ�ѧʽ��
��2���¶�Ϊ570�����յõ��ĺ�ɫ��ĩ��CuO��������������ΪSO3����Ӧ����ʽΪ��CuSO4
CuO+SO3����CuO��ϡ���ᷴӦ�IJ���������ͭ��ˮ������Ũ������ȴ�õ��ľ���ΪCuSO4?5H2O������ͼ���������ڵ����102�棻
��2���¶�Ϊ570�����յõ��ĺ�ɫ��ĩ��CuO��������������ΪSO3����Ӧ����ʽΪ��CuSO4
| ||
����⣺��1��CuSO4?5H2O���ȵ�102��ʱ��ʼ��ˮ�ֽ⣬113��ʱ�ɵõ����ȶ���һ���м����258��ʱ�Ż�����ֽ⣮��200��ʱʧȥ��ˮ������Ϊ0.80g-0.57g=0.23g��
���ݷ�Ӧ�Ļ�ѧ����ʽ��
CuSO4?5H2O
CuSO4?��5-n��H2O+nH2O
250 18n
0.80g 0.80g-0.57g=0.23g
=
�����n=4
200��ʱ�ù������ʵĻ�ѧʽΪCuSO4?H2O��
�ʴ�ΪCuSO4?H2O��
��2���¶�Ϊ570�����յõ��ĺ�ɫ��ĩӦ��CuO��������������ΪSO3����Ӧ����ʽΪ��CuSO4
CuO+SO3����CuO��ϡ���ᷴӦ�IJ���������ͭ��ˮ��
����Ũ������ȴ�õ��ľ���ΪCuSO4?5H2O������ڵ����102�森
�ʴ�Ϊ��CuSO4
CuO+SO3���� CuSO4?5H2O��102�棻
���ݷ�Ӧ�Ļ�ѧ����ʽ��
CuSO4?5H2O
| ||
250 18n
0.80g 0.80g-0.57g=0.23g
| 250 |
| 18n |
| 0.80g |
| 0.23g |
200��ʱ�ù������ʵĻ�ѧʽΪCuSO4?H2O��
�ʴ�ΪCuSO4?H2O��
��2���¶�Ϊ570�����յõ��ĺ�ɫ��ĩӦ��CuO��������������ΪSO3����Ӧ����ʽΪ��CuSO4
| ||
����Ũ������ȴ�õ��ľ���ΪCuSO4?5H2O������ڵ����102�森
�ʴ�Ϊ��CuSO4
| ||
���������⿼������ͭ�ᾧˮ�����IJⶨ���ܶȻ������ļ����Լ����ʵ���Ũ�ȵ��йؼ��㣬��Ŀ��Ϊ�ۺϣ�����ͼ����Ϣ����ɱ���Ŀ�Ĺؼ���

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
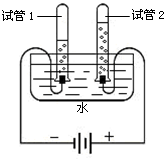
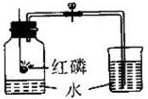 ��ͼ��ʾ�Dzⶨ����������������ʵ��װ�ã�
��ͼ��ʾ�Dzⶨ����������������ʵ��װ�ã�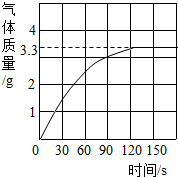 ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ���У�����CO2����������ͼ��ʾ����ʾ����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�� Mg��OH��2+2HCl�TMgCl2+2H2O
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ���У�����CO2����������ͼ��ʾ����ʾ����Ӧ�Ļ�ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2�� Mg��OH��2+2HCl�TMgCl2+2H2O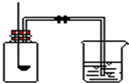 �ú����������������������IJⶨʵ�飬�ش��������⣮
�ú����������������������IJⶨʵ�飬�ش��������⣮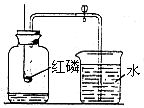 ��ͼ�Dzⶨ����������������ʵ��װ�ã������ʵ��ش����⣺
��ͼ�Dzⶨ����������������ʵ��װ�ã������ʵ��ش����⣺ ��ͼ��ʾijЩ���ʼ�ת����ϵ����Ӧ�����Ͳ��ֲ�����ʡ�ԣ�������A��EΪ�����������AΪ��ɫ��ĩ��B��D������ͬԪ����ɵ���ɫҺ�壬��B��������ɱ�����ã�C��Y��ZΪ��ɫ���壬����Y�ж���X������Ľ�������ش��������⣺
��ͼ��ʾijЩ���ʼ�ת����ϵ����Ӧ�����Ͳ��ֲ�����ʡ�ԣ�������A��EΪ�����������AΪ��ɫ��ĩ��B��D������ͬԪ����ɵ���ɫҺ�壬��B��������ɱ�����ã�C��Y��ZΪ��ɫ���壬����Y�ж���X������Ľ�������ش��������⣺