��Ŀ����
5��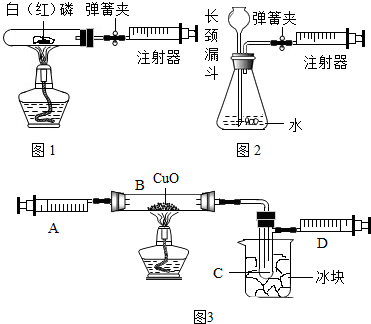 ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ��װ���г��ֵ�Խ��Խ�࣬����ijЩʵ������Ĺ۲��ʵ����̵ĸĽ����������벻����Ч��
ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ��װ���г��ֵ�Խ��Խ�࣬����ijЩʵ������Ĺ۲��ʵ����̵ĸĽ����������벻����Ч����1��ͼ1��120mL�Թ�����Ӧ��������ȼ�վ����ܱ���������У��ɷ�ֹ��Ⱦ��������250mLע�������������ȴ���100mL�̶ȴ���������ȼ�����ĵ����������
�������ټ��װ�õ������ԣ���װ��ҩƷ�������������ۼн����ɼУ����Ȱ��ף��۲��Թ���������������Ϊ����ȼ�գ������������̣���ȼ�ս������Թ���ȴ����ɼУ����Կ��������������Ƶ�Լ10mL�̶ȴ���ȡ����ֵ����
��2��ͼ2������ע���������ķ������Լ��װ�õ������ԣ�������������������ʱ������ܹ۲쵽D��ѡ����ţ�����˵��װ�����������ã�
A��ע��������Һ�� B��ƿ��Һ������
C������©����Һ������ D������©���¶˹ܿڲ�������
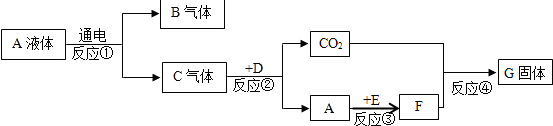
��3��ijѧ��Ϊ�˲ⶨ������Ԫ���γɵ���̬������X���ܶȱȿ���С������ɣ�������ͼ3��ʾ��ʵ�飮����ע����A�е�����X��������װ��CuO��Bװ�ã�ʹ֮��ȫ��Ӧ���õ����½����
��ʵ��ǰB�ܼ�ҩƷ������Ϊ21.32g��ʵ���Ϊ21.16g��
��C�����ռ��������ʵ���õ�H2��O2����D���ռ�������N2��
��X����Ԫ�ص���������14��3���ʣ�
����C���ռ�����Һ�壬������0.18g��
������ʵ���п���B�е������Ǻ�ɫ�����죮
����B�з�Ӧ�Ļ�ѧ����ʽ��3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$3H2O+3Cu+N2��
���� ��1������������Ӧ��ʵ�������ǣ�����ȼ�գ������������̣���������Լռ���������20%������ȼ�պ��Թ�������Լ����20%����10mL������ע��������Ӧ�����ƶ�10mL��
��2�����װ��������ʱ��ע������������������ƿ������ѹǿ��С��������ѹ�������س���©��ѹ����ƿ�У��ڳ���©���¶˻ῴ��������ð����
��3������ˮͨ��ֽ����������������������غ㶨�ɵ�֪ʶ���з������
��� �⣺��1�����Ȱ��ף��۲��Թ��������������ǣ�����ȼ�գ���������������ռ�������������Ϊ20%������ע��������Ӧ�����ƶ���10mL����
��2��ע������������������ƿ������ѹǿ��С��������ѹ�������س���©��ѹ����ƿ�У��ڳ���©���¶˻ῴ��������ð������ѡD��
��3����ʵ��ǰB�ܼ�ҩƷ������Ϊ21.32g��ʵ���Ϊ21.16g���ʼ��ٵ���Ԫ�ص�����Ϊ��21.32g-21.16g=0.16g��
��C�����ռ��������ʵ���õ�H2��O2����C���ռ�������ˮ��˵��X�к�����Ԫ�أ���D���ռ�������N2�����X�к��е�Ԫ�غ���Ԫ�أ�
�����ɵ�ˮ������Ϊx
H2+CuO$\frac{\underline{\;\;��\;\;}}{\;}$Cu+H2O��m
18 16
x 0.16g
$\frac{18}{16}=\frac{x}{0.16g}$
x=0.18g
����Ԫ�ص�����Ϊ0.18g-0.16g=0.02g
��X����Ԫ�ص���������14��3���������ǿɵõ���������Ԫ�ص�ԭ�Ӹ�����Ϊ1��3�����Ի�ѧʽΪNH3������ͭ���백�����ȷ�Ӧ����ͭ��ˮ�͵�����
�ʴ�Ϊ����1������ȼ�գ������������̣�10��
��2��D��
��3������0.18��
������ɫ�����죮
����3CuO+2NH3$\frac{\underline{\;\;��\;\;}}{\;}$3H2O+3Cu+N2��
���� ����������ʵ�鷽�����̵�̽��������̽��ʵ��Ҫ����������������������ƵIJ����в�ͬ������ȥ˼����ȥ̽�����Ӷ��ش���Ŀ���������⣮�йصļ���Ҫȷ����������Ҫ������ʵ�����У�

| ��� | ���� | ����һ | ������ |
| A | һ����̼�Ͷ�����̼ | ����ζ | ͨ��ʯ��ˮ |
| B | ����狀�̼��� | ����ɫ | ��ˮ�ܽ� |
| C | ʳ��ˮ��ҽ�þƾ� | ����ζ | �����ᾧ |
| D | ͭ�ۺ������������� | �ô������� | ��ϡ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | Na2O | B�� | CaO | C�� | SO3 | D�� | CuSO4 5H2O |
| A�� | ���¯������pH=3 | B�� | ��ò�������pH=13 | ||
| C�� | ��������һ��ʹ��Ч������ | D�� | ��������ʹ��ɫʯ����Һ��� |
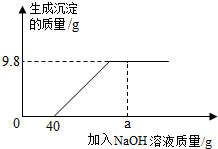 ���к�HCl��CuCl2�Ļ����Һ65.3g�������Һ����μ���������������Ϊ10%��NaOH��Һ�����ɳ��������������NaOH��Һ��������ϵ��ͼ��ʾ��
���к�HCl��CuCl2�Ļ����Һ65.3g�������Һ����μ���������������Ϊ10%��NaOH��Һ�����ɳ��������������NaOH��Һ��������ϵ��ͼ��ʾ��