��Ŀ����
10�����ʯ�������и��������ʯ����������ʯ����Ӳ�ȴ�����ʣ������ڽ��ʯ���������ʣ���ʯī�ķ�ĩ������ķ�ĩ��Ͽ��Ƴ�Ǧ��о���ɴ˿��Ƴ�ʯī���������ʣ���ɫ���ɫ��״̬���壬Ӳ��������ʯī�����е����ԣ���˳�������صĵ缫��ʯī�ͽ��ʯ������̼Ԫ����ɣ�����²��������ʲ����ԭ�������̼ԭ�ӵ����з�ʽ��ͬ���������ʵĽṹ�������ʣ��������־������ʵ���;������ ���ʵĽṹ�������ʵ����ʣ����ʵ����ʾ������ʵ���;������̼���ʵĽṹ�����ʺ���;�����ش�
��� �⣺���ʯ�������и��������ʯ����������ʯ����Ӳ�ȴ�����ʣ������ڽ��ʯ���������ʣ���ʯī�ķ�ĩ������ķ�ĩ��Ͽ��Ƴ�Ǧ��о���ɴ˿��Ƴ�ʯī���������ʣ���ɫ�����ɫ��״̬�ǹ��壬Ӳ��������ʯī�����е����ԣ���˳�������صĵ缫��ʯī�ͽ��ʯ������̼Ԫ����ɣ�����²��������ʲ����ԭ�������̼ԭ�ӵ����з�ʽ��ͬ���������ʵĽṹ�������ʣ��������־������ʵ���;��
�ʴ�Ϊ��Ӳ�ȴ����������ɫ�����壬���������磬̼ԭ�ӵ����з�ʽ��ͬ��
���� ������ѶȲ����˽�̼���ʵĽṹ�����ʵ�֪ʶ���ɷ������

��ϰ��ϵ�д�
 �ƸԴ��ž�ϵ�д�
�ƸԴ��ž�ϵ�д�
�����Ŀ
1���������ṹʾ��ͼ����ʾ�����ӵ��ǣ�������
| A�� |  | B�� |  | C�� |  | D�� |  |
5��ͨ������ʵ�飬�жϽ���X��Y��Z�Ļ���˳������X��Y��Z�ֱ�Ͷ��������ϡ�����У�ֻ��X��Z�ܷ�����Ӧ�����ų���������ZͶ��X����������Һ�У��н���X������X��Y��Z�Ļ˳��Ϊ��������
| A�� | Z��X��Y | B�� | X��Z��Y | C�� | Z��Y��X | D�� | Y��Z��X |
15������ͼʾ�ǻ�ѧʵ��Ļ���������������ȷ���ǣ�������
| A�� |  ��ȼ�ƾ��� | B�� |  ���� | C�� |  ��ȡҺ����� | D�� |  ��ȡ�������� |
2�������������г��������ʣ������ϳɷֵ�Ħ�����������������ݼ��ȣ�����Ħ�����������кܶ࣬��CaCO3��Al��OH��3��SiO2����Щ���ʵĻ���ij��ѧ��ȤС��ͬѧ��̽������Ħ�����ijɷ֣�
ʵ��̽��һ��ijƷ���������Ƿ���CaCO3��
[��������]�������裨SiO2������ϡ���ᷴӦ��
[���ʵ��]
ʵ��̽�������ⶨ��Ʒ��������CaCO3������������
��ȤС��ͬѧȡ��Ʒ��������Ʒ8.00g������������ϡ���ᣬ������ͼ1��ʾ��ʵ��װ�ý���ʵ�飬������װ�÷��ڵ�����ƽ�ϣ���������ƽ���������ݻ����ͼ2��
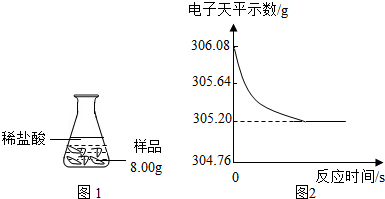
[���ݴ���]ʵ����������������0.88g�����Ʒ��������CaCO3��������Ϊ25%��
[�����뷴˼]
��1��д����ƿ�з�Ӧ�Ļ�ѧ����ʽCaCO3+2HCl�TCaCl2+H2O+CO2����дһ�����ɣ���
��2�����������Ҫ��������������CaCO3������������ƫС��ѡ�ƫ�������䡱��ƫС������
��3�������������ж�ϡ�����ѹ����ķ���ȡ��Ӧ����ƿ�е�������Һ���Թ��У��μ���ɫʯ����Һ����Һ���ɫ��д��ʵ�鲽�������
��4��ͬѧ�Ǿ�������һ����Ϊ������ͼ1װ�ô��ڿ�ѧ�����⣬�������ʵ��������˵�������������һ����������ӷ���
ʵ��̽��һ��ijƷ���������Ƿ���CaCO3��
[��������]�������裨SiO2������ϡ���ᷴӦ��
[���ʵ��]
| ʵ�鲽�� | ʵ������ | ʵ����� |
| ��ȡ������Ʒ���Թ��У��μ�ϡ���ᣮ | ��������ð�� | ��Ʒ�������к���CaCO3�� |
| �ڽ�����������ͨ�����ʯ��ˮ�� | ��ʯ��ˮ����� |
��ȤС��ͬѧȡ��Ʒ��������Ʒ8.00g������������ϡ���ᣬ������ͼ1��ʾ��ʵ��װ�ý���ʵ�飬������װ�÷��ڵ�����ƽ�ϣ���������ƽ���������ݻ����ͼ2��

[���ݴ���]ʵ����������������0.88g�����Ʒ��������CaCO3��������Ϊ25%��
[�����뷴˼]
��1��д����ƿ�з�Ӧ�Ļ�ѧ����ʽCaCO3+2HCl�TCaCl2+H2O+CO2����дһ�����ɣ���
��2�����������Ҫ��������������CaCO3������������ƫС��ѡ�ƫ�������䡱��ƫС������
��3�������������ж�ϡ�����ѹ����ķ���ȡ��Ӧ����ƿ�е�������Һ���Թ��У��μ���ɫʯ����Һ����Һ���ɫ��д��ʵ�鲽�������
��4��ͬѧ�Ǿ�������һ����Ϊ������ͼ1װ�ô��ڿ�ѧ�����⣬�������ʵ��������˵�������������һ����������ӷ���


 С�����������ᡢ��������������ͭ��̼�������������滯ѧƴͼ��Ϸ����ͼ������Ϸ����Ҫ��ͼ����������֮���ܷ�����Ӧ������C���ʵ���Һ����ɫ��A��E��Ӧ�����������ǹ�����õ�ԭ��֮һ��
С�����������ᡢ��������������ͭ��̼�������������滯ѧƴͼ��Ϸ����ͼ������Ϸ����Ҫ��ͼ����������֮���ܷ�����Ӧ������C���ʵ���Һ����ɫ��A��E��Ӧ�����������ǹ�����õ�ԭ��֮һ��