��Ŀ����
18��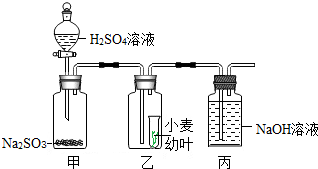 ����г���һЩ�����̷�ʹ�õ��ж���SO2�����ijЩʳƷ����Ư�ף�ij�Ƽ���ȤС����С����ҶΪʵ������о�SO2��ֲ���Σ��������ͨ���������ϣ���֪������Һ���̬���������Ʒ�Ӧ�ɲ���SO2���壺Na2SO3+H2SO4�TNa2SO4+SO2��+H2O���ʵ����ͼ��ʾ��
����г���һЩ�����̷�ʹ�õ��ж���SO2�����ijЩʳƷ����Ư�ף�ij�Ƽ���ȤС����С����ҶΪʵ������о�SO2��ֲ���Σ��������ͨ���������ϣ���֪������Һ���̬���������Ʒ�Ӧ�ɲ���SO2���壺Na2SO3+H2SO4�TNa2SO4+SO2��+H2O���ʵ����ͼ��ʾ����1����С���ʵ����ȡ������������Ϊ75%������Һ200�ˣ�������150�ˣ�
��2����С��������ȡ0.64�˶�������������Ҫ10%������������Һ���ٿˣ�
���� ��1������Һ�����������������������������������ļ��㹫ʽ���Լ��������������
��2�������ɶ���������������ݻ�ѧ����ʽ���Լ�����μӷ�Ӧ������������Һ��������
��� �⣺��1�������������Ϊ200g��75%=150g�����150��
��2������Ҫ����������Һ������Ϊx��
Na2SO3+H2SO4=Na2SO4+SO2��+H2O
126 64
x��10% 0.64g
$\frac{126}{x��10%}=\frac{64}{0.64g}$
���x=12.6g
����Ҫ����������Һ������Ϊ12.6g��
���� ���⿼���������������ļ��㡢�йػ�ѧ����ʽ�ļ��㡢��Ļ�ѧ���ʡ�ʵ��̽�����������Σ����֪ʶ���Ѷ��Դ�

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
13����֪Ũ�����ϡ������ܶȴ����������ֱ�Ϊ85%��5%������������Һ�������Ϻ�������Һ����������Ϊ��������
| A�� | С��45% | B�� | ����45% | C�� | ����45% | D�� | ��ȷ�� |
3�����б仯�У����ڻ�ѧ�仯���ǣ�������
| A�� | ��ˮ�ӷ� | B�� | ú������ | C�� | ��̥��ը | D�� | �ɱ����� |
10����ȥ���������е��������ʣ���ѡ�õ��Լ�������������������������ȷ���ǣ�������
| ѡ�� | ���ʣ�������Ϊ���ʣ� | �Լ� | �������� |
| A | CaO ��CaCO3�� | -- | ���ȵ��������ټ��� |
| B | CO2���壨HCl�� | ����������Һ��Ũ���� | ϴ�������� |
| C | NaCl���壨BaSO4�� | ˮ | �ܽ⡢���ˡ����� |
| D | NaCl���壨MgCl2�� | NaOH��Һ | ���ˡ����� |
| A�� | A | B�� | B | C�� | C | D�� | D |
8������Ǽ��ּ���������
���ݱ�����Ϣ���ش��������⣮
��1����ʢ����������ƯҺ�����Թ��У��������������̣�������Ӧ�Ļ�ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��2�����ܵ�ͨ����ʹ�����Ĺܵ���ͨ���裬��ʹ��ʱ��ֹ��Ƥ���Ӵ�����ԭ����NaOH����ǿ�ҵĸ�ʴ�ԣ�Ҳ�����롰����顱���ʹ�ã���ԭ����NaOH+HCl�TNaCl+H2O���û�ѧ����ʽ��ʾ����
| ���� |  ��ƯҺ |  ����� |  �ܵ�ͨ |
| �� �� | Ưϴ���ʹɫ������ | ����۹�������ζ | �ٵ���ͨ�������� |
| ��Ч�ɷ� | �������� | ���ᣨHCl�� | �������� |
��1����ʢ����������ƯҺ�����Թ��У��������������̣�������Ӧ�Ļ�ѧ����ʽΪ2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��2�����ܵ�ͨ����ʹ�����Ĺܵ���ͨ���裬��ʹ��ʱ��ֹ��Ƥ���Ӵ�����ԭ����NaOH����ǿ�ҵĸ�ʴ�ԣ�Ҳ�����롰����顱���ʹ�ã���ԭ����NaOH+HCl�TNaCl+H2O���û�ѧ����ʽ��ʾ����
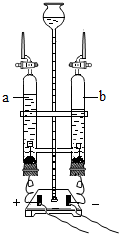 ˮ����������ͻ�ѧʵ��������ʮ����Ҫ�����ã�
ˮ����������ͻ�ѧʵ��������ʮ����Ҫ�����ã�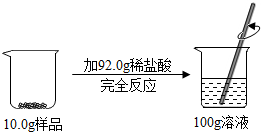 ij��Ʒ������ͭ��ͭ��ɣ�ȡ10.0g����Ʒ���ձ��У������м���92.0gϡ���ᣬǡ����ȫ��Ӧ������������Һ����Ϊ100.0g������ѧ��Ӧ����ʽΪ��CuO+2HCl�TCuCl2+H2O��
ij��Ʒ������ͭ��ͭ��ɣ�ȡ10.0g����Ʒ���ձ��У������м���92.0gϡ���ᣬǡ����ȫ��Ӧ������������Һ����Ϊ100.0g������ѧ��Ӧ����ʽΪ��CuO+2HCl�TCuCl2+H2O��