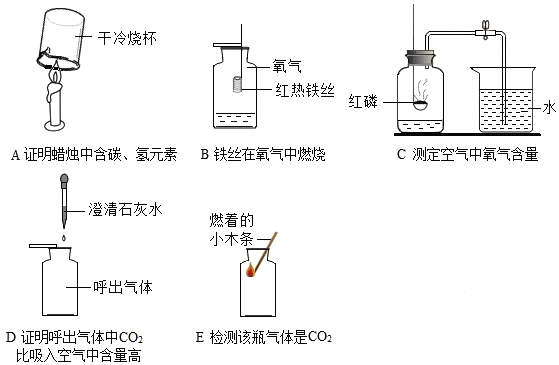
��Ŀ����
6��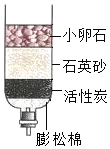 ˮ������ͨ�����������֮һ��
ˮ������ͨ�����������֮һ����1�������ǵĺ�ˮ����ͼ��ʾ�ļ���ˮ�����о���������С��ʯ��ʯӢɰ�������ǹ��ˣ��ô�װ�þ�����õ���ˮ���ڻ�����������������
��2�����ˮʵ���֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2����
��3��������ˮϴ�·�ʱ��������������ĭ�Ҳ���������˵��������ˮ��Ӳˮ���Ӳˮ������ˮ������
���� ��1��С��ʯ�������ǹ������е�ijЩ��IJ��������ʣ�ͨ����װ�þ�����õ���ˮ����Ȼ����һЩ����ˮ�����ʣ�
��2�����ݵ��ˮʵ��ķ�Ӧԭ���������
��3������Ӳˮ�����ˮ��ϲ��������ĸ�������ˮ�����ˮ��ϲ����϶����ĭ���
��� �⣺��1�������ǵĺ�ˮ����ͼ��ʾ�ļ���ˮ�����о�������������С��ʯ��ʯӢɰ�������ǹ������е�ijЩ���������ʣ��ô�װ�þ�����õ���ˮ��Ȼ����һЩ���������ʣ����ڻ���
��2�����ˮ����������������ʵ���֤��ˮ���⡢������Ԫ����ɣ��÷�Ӧ�Ļ�ѧ����ʽΪ��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2����
��3����ˮ�м������ˮʱ�����������������ĭ��˵������ˮ�������������ĭ���ٻ�����ĭ��˵����Ӳˮ������ˮϴ�·�ʱ��������������ĭ�Ҳ���������˵��������ˮ��Ӳˮ��
�𰸣���1�����ˣ� ������2��2H2O$\frac{\underline{\;ͨ��\;}}{\;}$2H2��+O2������3��Ӳˮ��
���� ������Ҫ���龻��ˮ�ķ��������ʱҪ��������Լ��ˮ�����壮

��ϰ��ϵ�д�
 ֥�鿪���γ�������ϵ�д�
֥�鿪���γ�������ϵ�д�
�����Ŀ
16��ʵ�����ò��ᣨH2C2O4����ȡCO�Ļ�ѧ����ʽΪH2C2O4$\frac{\underline{\;һ������\;}}{\;}$CO��+X+CO2������X�Ļ�ѧʽΪ��������
| A�� | H2O2 | B�� | H2O | C�� | CH4 | D�� | H2 |
11�����µ�ƽ�͵����ֻ�Ʈ�㣬���ŵ������ԭ���ǣ�������
| A�� | ���ӵ�������С | B�� | ���Ӽ��м�� | C�� | �����ڲ����˶� | D�� | ������ԭ�ӹ��� |
15�����з����м��ܱ�ʾ���ʵ�Ԫ����ɣ����ܱ�ʾ���ʵ�һ�����ӵ��ǣ�������
| A�� | Cl | B�� | Cu | C�� | H2O2 | D�� | NaCl |
 ͼ��ΪX��Y��Z���ֹ������ʵ��ܽ�����ߣ��������߿�֪30oCʱX���ܽ��Ϊ20g����40oCʱ��С��ͬѧ������100��28%��Y���ʵ���Һ�����ܣ���ܡ����ܡ����ﵽĿ�ģ�
ͼ��ΪX��Y��Z���ֹ������ʵ��ܽ�����ߣ��������߿�֪30oCʱX���ܽ��Ϊ20g����40oCʱ��С��ͬѧ������100��28%��Y���ʵ���Һ�����ܣ���ܡ����ܡ����ﵽĿ�ģ�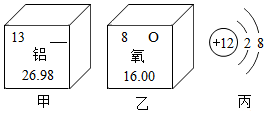 ��ͼ��ʾΪijЩԪ�غ�ԭ�ӽṹ�IJ�����Ϣ��
��ͼ��ʾΪijЩԪ�غ�ԭ�ӽṹ�IJ�����Ϣ��