��Ŀ����
1��Ϊ�Ӷ����Ƕ���ʶ�кͷ�Ӧ��С��ͬѧ������Ʋ�����������ʵ�飺����С�ձ��е���8%������������Һ10g������2�η�̪�Լ�����Һ�ʺ�ɫ������ȡ10%��ϡ������εμӵ�����������Һ�У��ߵα�����������룬��Һ��ɫ��dz��������Һ��ɫ��ʧ˲�䣬ֹͣʵ�飮��ֹͣʵ��ʱ������Һǡ�÷�Ӧ�������С��ʵ���ش�
��1��ʵ���з�̪�Լ����������жϷ�Ӧ���кͽ��еij̶ȣ�
��2���������ʱ����ȥϡ����������Ƕ��٣�����ȷ��0.1��
���� ��1����̪������ɫ�������ɫ�����ݷ�̪��ɫ�ı仯ȷ����Һ���е������
��2�����ݻ�ѧ����ʽ�е���Է��������ı�ֵ�����ñ�����ϵ������⣮
��� �⣺��1����̪������ָʾ����Եģ����ݷ�̪��ɫ�ı仯�������ж��кͷ�Ӧ���е������
��2������ȥϡ���������Ϊx��
NaOH+HCl=NaCl+H2O
40 36.5
10g��8% x��10%
$\frac{40}{10g��8%}$=$\frac{36.5}{x��10%}$
x=7.3g
����ȥϡ�����������7.3g��
�ʴ�Ϊ����1���жϷ�Ӧ���кͽ��еij̶ȣ�
��2����ȥϡ�����������7.3g��
���� ������Ҫ�Ӷ����ĽǶȶ��кͷ�Ӧ�������о���Ҫ���ջ�ѧ����ʽ�ļ��㣮

��ϰ��ϵ�д�
�����Ŀ
11�����з�Ӧ���ڸ��ֽⷴӦ���ǣ�������
| A�� |  | B�� |  | ||
| C�� |  | D�� |  |
13����ͼ��ʾʵ���������ȷ���ǣ�������
| A�� | 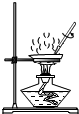 ���� | B�� |  ȡ�ù����ĩ | C�� | 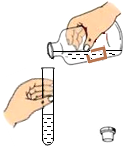 ȡ��Һ�� | D�� | 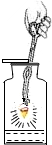 ��˿��O2��ȼ�� |

 ������Τ�Ȼ���ע��Һ������H7N9���������е���Чҩ��֮һ��������Τ�Ļ�ѧʽ��C15H28NxO4����
������Τ�Ȼ���ע��Һ������H7N9���������е���Чҩ��֮һ��������Τ�Ļ�ѧʽ��C15H28NxO4���� ��
�� �γɵĻ�����Al2O3��
�γɵĻ�����Al2O3��