��Ŀ����
��֪�����Ȼ�ѧ����ʽ��
��H2(g)��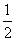 O2(g)===H2O(l) ��H����285.8 kJ��mol��1
O2(g)===H2O(l) ��H����285.8 kJ��mol��1
��H2(g)��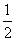 O2(g)===H2O(g) ��H����241.8 kJ��mol��1
O2(g)===H2O(g) ��H����241.8 kJ��mol��1
��C(s)��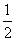 O2(g)===CO(g) ��H����110.5 kJ��mol��1
O2(g)===CO(g) ��H����110.5 kJ��mol��1
��C(s)��O2(g)===CO2(g) ��H����393.5 kJ��mol��1
�ش����и����⣺
(1)������Ӧ�����ڷ��ȷ�Ӧ����__________________________________________��
(2)H2��ȼ����Ϊ________��C��ȼ����Ϊ________��
(3)ȼ��10 g H2����Һ̬ˮ���ų�������Ϊ________��
(4)CO��ȼ����Ϊ________�����Ȼ�ѧ����ʽΪ______________��
�𰸡�(1)�٢ڢۢܡ�(2)��285.8 kJ��mol��1����393.5 kJ��mol��1��(3)1 429.0 kJ��(4)��283.0 kJ��mol��1 CO(g)��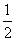 O2(g)===CO2(g)����H����283.0 kJ��mol��1
O2(g)===CO2(g)����H����283.0 kJ��mol��1
������(1)����ȼ�վ�Ϊ���ȷ�Ӧ���٢ڢܾۢ�Ϊ���ȷ�Ӧ��
(2)ȼ����Ϊ1 mol����ȼ�������ȶ�������ʱ�ų���������H2��ȼ����Ϊ��285.8 kJ��mol��1��C��ȼ����Ϊ��393.5 kJ��mol��1��
(3)Q���� ��285.8 kJ��mol��1��1 429.0 kJ��
��285.8 kJ��mol��1��1 429.0 kJ��
(4)�ɢܣ��ۿɵã�CO(g)��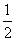 O2(g)===CO2(g)
O2(g)===CO2(g)
��H����393.5 kJ��mol��1��110.5 kJ��mol��1����283.0 kJ��mol��1����ȼ����Ϊ��283.0 kJ��mol��1��

 B��2Al+3H2SO4=Al2(SO4)3+3H2��
B��2Al+3H2SO4=Al2(SO4)3+3H2��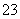 )��c(HCO
)��c(HCO )��c(H2CO3)
)��c(H2CO3)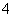 )��c(H��)��c(OH��)
)��c(H��)��c(OH��) O2(g)===2CO2(g)��H2O(l)
O2(g)===2CO2(g)��H2O(l)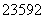 U��
U��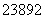 U���˵�������Ҫ��ͬλ�ء�
U���˵�������Ҫ��ͬλ�ء�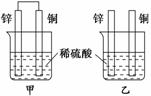 ����пƬ�ʹ�ͭƬ��ͼʾ��ʽ����ͬŨ�ȵ�ϡ������һ��ʱ�䣬����������ȷ����
����пƬ�ʹ�ͭƬ��ͼʾ��ʽ����ͬŨ�ȵ�ϡ������һ��ʱ�䣬����������ȷ����