��Ŀ����
19����������茶��廯ѧʽΪxFeSO4•y��NH4��2SO4•zH2O������ҩ����Ʒ����й㷺��Ӧ�ã����ϣ�����������茶���������ˮ���������Ҵ�������������茶�������ʱ����200������ֻ�нᾧˮʧȥ��

��̽��һ�����Ʊ���������茶��壺ѧ���ú�������ͭ�ķ���м�Ʊ���������茶��壬������ͼ1��
��1����ʢ�з���м�������У�������ˮ��ϴ�Ӽ�����ֽ��裮�Գ�ȥ����м��������ۣ���������ϴ�Ӽ����黯���ã��������з�����Ӧ�Ļ�ѧ����ʽΪFe+H2SO4=FeSO4+H2����
��2����������IJ����������ձ�����������©�������˲�������ֽ�ж����۷���Ϊ�˼ӿ�������ʣ���ѡ���۷���A����3���������й���ʱ��Ҫ���Ƚ���˵�����¶ȸߣ�FeSO4�ܽ�ȴ��С�������˺�õ��Ĺ�����һ�����е�������Cu��
��4�������ڵ�Ŀ���ǵõ���������茶��壮����˳���ǣ�b��c��a��d��
a������ b������Ũ�� c����ȴ�ᾧ d��ϴ�Ӹ���
��5����������ʹ���Ҵ�ϴ�ӣ��ɿ������ɣ����������ŵ���AB��
A��������ˮϴ������ɵľ������ B���ƾ��ӷ����ɵ������ɾ���
��̽��������̽����������淋����
��ʵ�鲽�衿��ȡ2�ݵ���������������茶��壬�ֱ����ʵ�飺��һ���м��������NaOH��Һ����ַ�Ӧ��õ�0.68g NH3������һ���м��������BaCl2��Һ����ַ�Ӧ����ˡ�ϴ�ӡ�����õ�9.32g�������ش��������⣺
��6��ʵ�������ɵ�NH3��ʹʪ��ĺ�ɫʯ����ֽ������д����NH4��2SO4��NaOH��Ӧ�Ļ�ѧ����ʽ����NH4��2SO4+2NaOH=Na2SO4+2NH3��+2H2O��
��7��ȡ���һ��ϴ��Һ������Na2SO4��Һ������������ɫ��������˵�������Ѿ�ϴ�Ӹɾ�������ʵ�����ݣ���x ��y�ı�ֵ1��1����Է���������NH3-17��BaSO4-233����Ϊ��һ��ȷ����������茶������ɣ���ȡ7.84g��������茶��壬������ʹ��ֽ⣬ʣ������������¶ȵĹ�ϵ��ͼ3��ʾ������ͼʾ���ݣ���������茶����У�x��y��z=1��1��6��
��8��д��A�㵽B�㷢����Ӧ�Ļ�ѧ����ʽFeSO4•��NH4��2SO4•6H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeSO4+��NH4��2SO4•2H2O+4H2O��
���� ��1������ϴ�Ӽ����黯���÷���ϴ�Ӽ������ã���������ϡ���ᷴӦ��������������������д��Ӧ�ķ���ʽ��
��2�����ݹ��˵IJ��������������Ʒ����
��3�����ݸ���ͭ��������ᷴӦ�����Կ��Թ��˳���������ʵ��IJ������������������ܽ�ȣ�
��4�����������������ܽ�����¶ȵ�Ӱ������ᾧ�ķ�����
��5��������������茶���������ˮ�����ھƾ����ƾ������ʷ������
��6�����ݰ����ܺ�ˮ��Ӧ���ɰ�ˮ����ˮ�ʼ�����ʹʯ�����ɫ����NH4��2SO4��NaOH��Ӧ���������ơ�ˮ�Ͱ������ֽⷴӦ�Ķ�����
��7��֤���Ȼ�������ֻҪ֤����������ʣ�༴�ɣ�������������������ƺͺ��Ȼ����ķ�Ӧ��������x��y�ı�ֵ���ٽ�Ϸ����ķ�Ӧ���x��y��z�ı�ֵ��
��8��A��BʱxFeSO4•y��NH4��2SO4•zH2O�ֽ�õ�������李���������茶����ˮ�Ĺ��̣�
��� �⣺��1��������ˮ��ϴ�Ӽ�����ֽ��裬�Գ�ȥ����м��������ۣ���������ϴ�Ӽ����黯���ã�����ϡ���ᷴӦ����������������������Ӧ�Ļ�ѧ����ʽΪ��Fe+H2SO4=FeSO4+H2����
��2����������IJ����������ձ�����������©�������˲�������ֽ�ж����۷���Ϊ�˼ӿ�������ʣ���ѡ���۷���A��
��3������ϡ����������Ժ����ᷴӦ����������������������ͭ��������ᷴӦ�����ͭ�ͱ����˳��������ԣ����˺�õ��Ĺ�����һ�����е�������ͭ��
��4�������ڵ�Ŀ���ǵõ���������茶��壬������������淋��ܽ�����¶ȵ�Ӱ��ϴ����Ի����������裺����Ũ����Ȼ����ȴ�ᾧ���ٹ��ˣ����ϴ�Ӹ��
��5��������������ˮ�����ھƾ�����A����ϴ����������������ʧ�϶࣬���þƾ�ϴ�ӣ��ƾ����ӷ����ܵõ������Ĵ����������������壬�ʴ𰸣�AB��
��6�������ܺ�ˮ��Ӧ���ɰ�ˮ����ˮ�ʼ�����ʹʯ�����ɫ����NH4��2SO4��NaOH��Ӧ���������ơ�ˮ�Ͱ����� ��Ӧ�ķ���ʽ�ǣ�NH4��2SO4+2NaOH=Na2SO4+2NH3��+2H2O��
��7����������������������ɫ����֤���������Ѳ�����BaCl2����˵�������Ѿ�ϴ�Ӹɾ���
����������茶���������淋�����Ϊx��
��NH4��2SO4+2NaOH=Na2SO4+2NH3��+2H2O
132 34
x 0.68g
$\frac{132}{34}=\frac{x}{0.68g}$ ��ã�x=2.64g
�������立�Ӧ�������ᱵ����������Ϊy
��NH4��2SO4+BaCl2=BaSO4��+2NH4Cl
132 233
2.64g y
$\frac{132}{233}=\frac{2.64g}{y}$ ��ã�y=4.66g y=4.66g
���Ժ�����������Ӧ�������ᱵ����������Ϊ9.32g-4.66g=4.66g��
�������������Ӧ�������ᱵ����������Ϊz
FeSO4+BaCl2=BaSO4��+FeCl2
152 233
z 4.66g
$\frac{152}{233}=\frac{z}{4.66g}$ ��ã�z=3.04g
�����������$\frac{3.04g}{2.64g}$=$\frac{152x}{132y}$��$\frac{x}{y}$=$\frac{1}{1}$���ʴ𰸣�1��1��
��A��B������������������������ɵ�ˮ��������7.84g��������茶�����ˮ������Ϊ7.84g-5.68g=2.16g��$\frac{132y}{18z}$=$\frac{2.64g}{2.16g}$��$\frac{y}{z}$=$\frac{1}{6}$ �ʴ𰸣�1��1��6��
��8����FeSO4•��NH4��2SO4•6H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeSO4+��NH4��2SO4•2H2O+4H2O������FeSO4•��NH4��2SO4•6H2O�ֽ�õ���������������李���ˮ����Ӧ�ķ���ʽ�ǣ�FeSO4•��NH4��2SO4•6H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeSO4+��NH4��2SO4•2H2O+4H2O��
�ʴ�Ϊ����1���黯��Fe+H2SO4=FeSO4+H2������2��©����A����3����Cu����4��c��a����5��AB����6����NH4��2SO4+2NaOH=Na2SO4+2NH3��+2H2O����7��1��1��1��1����8��FeSO4•��NH4��2SO4•6H2O$\frac{\underline{\;\;��\;\;}}{\;}$FeSO4+��NH4��2SO4•2H2O+4H2O��
���� ͨ�����������ѧ���۲���ͼ���������Լ��ȽϷ��������������⿼���˳��������������Լ�ijЩ�ε����ʣ���ɴ��⣬������������ṩ�����ݣ����г�ȡ���õ���Ϣ��������е�֪ʶ���У�������һ��ʵ��̽���⣬�ܽϺõĿ���ѧ�������ͽ������������������˳��������������Լ�ijЩ�ε����ʣ����ʱҪ��������ṩ��Ϣ��������֪ʶϸ�ķ������

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д� ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� �ڲⶨ����������������ʵ���У�Сǿ��������ͼ��ʾװ�ã���������ע������ɵ��ܱ�ϵͳ������25mL��������װ��ϸͭ˿�IJ����ܼ��ȣ�ͬʱ�����ƶ�����ע�������������������ڵ�ͭ˿�ڽϳ�ʱ������һ���仯ʱֹͣ���ȣ�����ȴ�����£�������ȫ������һ��ע�����ڣ��۲��ܱ�ϵͳ�ڿ�������仯��
�ڲⶨ����������������ʵ���У�Сǿ��������ͼ��ʾװ�ã���������ע������ɵ��ܱ�ϵͳ������25mL��������װ��ϸͭ˿�IJ����ܼ��ȣ�ͬʱ�����ƶ�����ע�������������������ڵ�ͭ˿�ڽϳ�ʱ������һ���仯ʱֹͣ���ȣ�����ȴ�����£�������ȫ������һ��ע�����ڣ��۲��ܱ�ϵͳ�ڿ�������仯����1����ʵ����ȹ����У����滺���ƶ�����ע����������Ŀ����ʹ�����е�������ַ�Ӧ��
��2��д����ʵ���з�Ӧ�Ļ�ѧ����ʽ2Cu+O2$\frac{\underline{\;\;��\;\;}}{\;}$2CuO��
��3��Сǿ���ʵ�������£�
| ��Ӧǰע��������������� | ��Ӧ��ע��������������� |
| 25mL | 22mL |
��û�н��滺���ƶ�����ע�����������ڶ���ʱû����ȴ�����£��ۼ���ͭ˿��̫�٣��ܼ���ͭ˿��̫��
��4��ͨ�������ʵ�飬��ѧ���IJ����������ij�ɷֺ����ķ�����ͨ����ѧ��Ӧ��ȥ������е�һ�ֳɷ֣��ٲ���������ڷ�Ӧǰ����������������ı仯���Ӷ��ó����ֳɷֵĺ�����
��5����ʵ�黹��˵�������Ļ�ѧ���ʲ�ȼ�գ���֧��ȼ�գ�
 ��Ԫ�����ڱ�����Ԫ�ص������Ϣ��ͼ��ʾ������˵����ȷ����C��
��Ԫ�����ڱ�����Ԫ�ص������Ϣ��ͼ��ʾ������˵����ȷ����C��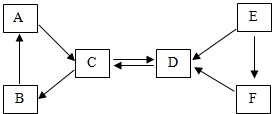 ȡA----F���ֳ������ʣ�����A�����ڸ�������������B�dz��õĹ���������A��B��C���嶼����ͬ�ֽ���Ԫ�أ�E�Ǻ�ɫ���嵥�ʣ�D��E��F������ͬ�ַǽ���Ԫ�أ����ǵ�ת����ϵ��ͼ��ʾ����ش�
ȡA----F���ֳ������ʣ�����A�����ڸ�������������B�dz��õĹ���������A��B��C���嶼����ͬ�ֽ���Ԫ�أ�E�Ǻ�ɫ���嵥�ʣ�D��E��F������ͬ�ַǽ���Ԫ�أ����ǵ�ת����ϵ��ͼ��ʾ����ش�