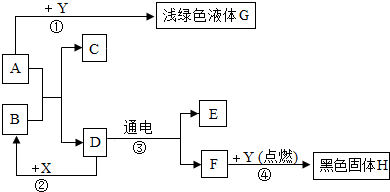
��Ŀ����
12��ijͬѧΪ̽��ij��ͭ���Ͻ������������������Ⱥ����ձ��н������Ĵ����飨���ʲ���ϡ���ᷴӦ����ʵ���������±���| ��һ�� | �ڶ��� | ������ | ���Ĵ� | |
| ��ȡ�Ͻ������/g | 10 | 10 | 20 | 30 |
| ����ϡ���������/g | 80 | 100 | 50 | X |
| ��Ӧ���ձ���ʣ�����ʵ�����/g | 89.8 | 109.8 | 69.8 | Y |
��1���ϱ����Ĵ�ʵ���кϽ������ǡ����ϡ������ȫ��Ӧ��������X=150��Y=179.4��
��2����ͭ���Ͻ�����������������
��3������ϡ���������������������������0.1%����
���� ��1�����ݱ���ǰ���鷴Ӧ�����ݽ��з�����
��2�������������ᷴӦ���������������������������е����ݼ�С���㣮
��� �⣺��1����һ�����ν�������10gʱ����������������Ϊ0.2g�������ν���20g����50gʱ����������������Ϊ0.2g������10g������50g��ǡ����ȫ��Ӧ�����Ե��Ĵν���30gʱ��������������Ϊ0.6g��X��150g��Y��179.4g��
��2����10g���Ͻ�����������Ϊx��50gϡ��������������Ϊy
Fe+H2SO4=FeSO4+H2��
56 98 2
x y 80g+10g-89.8g=0.2g
$\frac{56}{x}$=$\frac{98}{y}$=$\frac{2}{80g+10g-89.8g}$
x=5.6g
y=9.8g
ͭ���Ͻ���������������Ϊ$\frac{5.6g}{10g}$��100%=56%��
��3������ϡ����������������Ϊ��$\frac{9.8g}{50g}$��100%=19.8%��
�ʴ�Ϊ����1��150g��179.4g��
��2�����Ͻ���������������Ϊ56%��
��3��ϡ������������������Ϊ19.8%��
���� ������Ҫ�����˻�ѧ����ʽ�ļ��㣬�ѶȲ���ע�����Ĺ淶�Ժ�ȷ�ԣ�

��ϰ��ϵ�д�
�����Ŀ
14������ͼʾ��ʵ������в���ȷ���ǣ�������
| A�� |  ��������ζ | B�� |  �ƹ������� | C�� |  ��Һ����� | D�� |  ϡ��Ũ���� ϡ��Ũ���� |
3�����������ӾƼݵ�˾�����м��ʱ��һ���������»�ѧ��Ӧԭ����
C2H5OH+4CrO3���Ⱥ�ɫ��+6H2SO4�T2X����ɫ��+9H2O+2CO2����������X�Ļ�ѧʽΪ��������
C2H5OH+4CrO3���Ⱥ�ɫ��+6H2SO4�T2X����ɫ��+9H2O+2CO2����������X�Ļ�ѧʽΪ��������
| A�� | Cr2��SO4��3 | B�� | CrSO4 | C�� | Cr2O3 | D�� | CrSO3 |
4�������й�ũ��Ժʹ�û��ʵ�˵������ȷ���ǣ�������
| A�� | С������ֲ�˲��ˣ���������ƬҶ�ӷ��ƣ��������ж�Ӧʩ���ʵ����� | |
| B�� | С���ܽ�����ء���ۡ��Ȼ�識����ȿ���ɫ�ٽ�ʣ���ɫ����ֱ�����ʯ�һ����ĥ����ζ���� | |
| C�� | ����������Ӫ��Һ�к��и��Ϸʵ�K3PO4 | |
| D�� | С���ְ�Ϊ��ũ�����������ն�Ǯ����˵��ʩ���ʲ����ƻ������ˮ�����û��� |
2��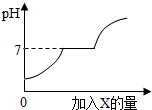 ���������CaCl2�Ļ����Һ����������μ��������ij����X����Һ��pH�����X�����ı仯��ϵ��ͼ��ʾ����X�����ǣ�������
���������CaCl2�Ļ����Һ����������μ��������ij����X����Һ��pH�����X�����ı仯��ϵ��ͼ��ʾ����X�����ǣ�������
 ���������CaCl2�Ļ����Һ����������μ��������ij����X����Һ��pH�����X�����ı仯��ϵ��ͼ��ʾ����X�����ǣ�������
���������CaCl2�Ļ����Һ����������μ��������ij����X����Һ��pH�����X�����ı仯��ϵ��ͼ��ʾ����X�����ǣ�������| A�� | ˮ | B�� | ������Һ | C�� | ̼��� | D�� | �ռ���Һ |