��Ŀ����
5����ͼͼʾ�ǵ�һ�µ�ѧϰ�н��й���ѧ��ʵ�����ʾ��ͼ����ش��������⣺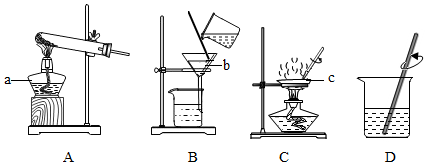
��1��д����Ŷ�Ӧ�����������ƣ�
a�ƾ��ƣ� b©���� c������
��2��ѧ�����д����ᴿ��ʵ����Ҫ�����в�����У�
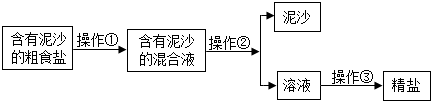
�����������У������١��ڡ���������װ��ͼ�е�DBC����A��Eѡ����գ���
�����������У������ڵ������ǹ��ˣ��ò��������������õ����������Dz����������ijͬѧ�øò����õ���Һ����Ȼ��Щ���ǣ���ԭ���������ֽ����ȣ�ֻ��дһ�㣩��
��ijС���ѧ��ʵ�����õľ��εIJ��ʣ�����=���þ��������/��ȡ���ε���������100%�����ϵͣ�����ܵ�ԭ����bc������ĸ����
a������ʱ��Һ�������ֽ��
b������ʱ���й��彦��
c���ܽ�ʱ����ˮ�����㵼���Ȼ��ƹ�����ʣ��
��3��ijͬѧ��Aװ�ý��м��ȸ�����ع����ʵ�飬��װ��Ҫ���Թܿ�Ҫ��������б��ԭ���Ƿ�ֹ����ˮ���������Թܵ�ը�ѣ��������ɵ������ô����ǵ�ľ������������������þ�������ڿ�����ȼ�գ�Ҳ���������Ĵ��ڣ���д��þ���ڿ�����ȼ�յ�����ҫ�۵İ⣬�ų��������ȣ����ɰ�ɫ���壮
���� ��1�����ݳ������������ƺ���;�����ش�
��2�����ݴ����ᴿ��ʵ�������衢ע����������ش��йص����⣻
��3�������ø��������ȡ������ע��������������ʺ�þȼ�յ���������ش�
��� �⣺��1����ͼʾ��֪����Ŷ�Ӧ�����������ƣ��ֱ��ǣ�a �Ǿƾ��ƣ� b ��©���� c ��������
��2�����ڽ��д����ᴿ��ʵ���У�����Ҫ�����ν����ܽ⣬�ٽ��й��ˣ����ͨ�������õ����Σ����ԣ������١��ڡ���������װ��ͼ�е�DBC��
��������������֪�����������в����ڵ������ǹ��ˣ��ò��������������õ����������� �����������ijͬѧ�øò����õ���Һ����Ȼ��Щ���ǣ���ԭ���������ֽ�����������ɾ��ȣ�
��a������ʱ��Һ�������ֽ�ߣ��ᵼ�����ʽ���ʳ���л����ʳ�������࣬����ƫ�ߣ�
b������ʱ���й��彦�����ᵼ��ʳ���е������٣�����ƫ�ͣ�
c���ܽ�ʱ�������ˮ������ᵼ��ʳ��ûȫ�����ܽ⣬�����в���ʳ�α����˳�ȥ�����¼��٣�����ƫ�ͣ�
��3��ijͬѧ��Aװ�ý��м��ȸ�����ع����ʵ�飬��װ��Ҫ���Թܿ�Ҫ��������б��ԭ���Ƿ�ֹ����ˮ���������Թܵ�ը�ѣ��������ɵ������ô����ǵ�ľ������������������þ�������ڿ�����ȼ�գ�Ҳ���������Ĵ��ڣ�þ���ڿ�����ȼ�յ������ǣ�����ҫ�۵İ⣬�ų��������ȣ����ɰ�ɫ���壮
�ʴ�Ϊ����1���ƾ��ƣ� ��©���� ��������
��2����DBC�����ˣ�����������ֽ����ȣ���bc��
��3����ֹ����ˮ���������Թܵ�ը�ѣ��ô����ǵ�ľ��������������ҫ�۵İ⣬�ų��������ȣ����ɰ�ɫ���壮
���� �ڽ������ʱ������Ҫ�������ֲ�����������ã�Ȼ����ѡ���е�˳����з������

| A�� | 1mL��2mL | B�� | 0.5mL | C�� | 3mL��4mL | D�� | 5mL��6mL |
| A�� | ͨ������£���������ɫ����ζ������ | |
| B�� | ͨ�����¼�ѹ����ʹ����Һ��Ϊ����ɫ��Һ�� | |
| C�� | �����ɹ�����ֲ����� | |
| D�� | ������һ�����ʱȽϻ��õ����壬�������������� |
| A�� |  | B�� |  | C�� |  | D�� |  |
| ʵ����� | �� | �� | �� |
| �Ͻ��������ˣ� | 0.51 | 0.70 | 0.90 |
| �����������ˣ� | 0.05 | 0.06 | 0.06 |
��2���Ͻ���þ�����������ȣ�
��3���������������������
 ��ͼ�ڲⶨ�����������ĺ���ʵ���У�ʵ�������ǣ�
��ͼ�ڲⶨ�����������ĺ���ʵ���У�ʵ�������ǣ�