��Ŀ����
 С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮ʵ���������ݼ��±�����������������йؼ��㣺
| �ձ��� | �ձ��� | �ձ��� | |
| ����10.0%�Ȼ�����Һ��������g�� | 55.5 | 120.0 | 150.0 |
| ��ɫ�����������g�� | 5.0 | 10.0 | 10.0 |
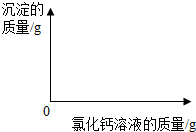
��2����ͼ�л��Ƴ���ɫ����������Ȼ�����Һ��������ϵͼ��ͼ��Ҫע����ɫ�������Ȼ�����Һ�������������
��3����ַ�Ӧ���ձ������Ȼ��Ƶ��������������Ƕ��٣�
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���,�й��������������ļ���
ר�⣺�ۺϼ��㣨ͼ���͡������͡��龰�ͼ����⣩
��������1�����������е����ݿ���֪��11.0g��Ʒ�������10.0g���������Ծݴ˽��̼���ƺ��Ȼ��Ʒ�Ӧ�Ļ�ѧ����ʽ���������̼���Ƶ�������
��2������ͼ����Ϣ����֪���Ƚ��ձ�����ձ����е����ݿ���֪����55.5g�Ȼ�����Һ���Ժ�̼���Ʒ�Ӧ����5.0g��������ô����10.0g�����������Ȼ�����Һ������Ϊ111.0g�����Ծݴ���ͼ������ɽ��
��3���ձ������Ȼ��ư���ԭ������л��еĺ�̼�������Ȼ��Ʒ�Ӧ���ɵģ��ٸ��������غ㶨�ɺ����������������㼴�ɣ�
��2������ͼ����Ϣ����֪���Ƚ��ձ�����ձ����е����ݿ���֪����55.5g�Ȼ�����Һ���Ժ�̼���Ʒ�Ӧ����5.0g��������ô����10.0g�����������Ȼ�����Һ������Ϊ111.0g�����Ծݴ���ͼ������ɽ��
��3���ձ������Ȼ��ư���ԭ������л��еĺ�̼�������Ȼ��Ʒ�Ӧ���ɵģ��ٸ��������غ㶨�ɺ����������������㼴�ɣ�
����⣺��ʵ�����ݿ�֪��11.0g��Ʒ�е�̼�������Ȼ�����Һ��ַ�Ӧ�������10.0g̼��ƣ�
��1����Ҫ����10.0g̼�����Ҫ̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy
Na2CO3+CaCl2�TCaCO3��+2NaCl
106 100 117
x 10.0g y
=
=
��ã�x=10.6g y=11.7g
��2������ͼ����Ϣ����֪���Ƚ��ձ�����ձ����е����ݿ���֪����55.5g�Ȼ�����Һ���Ժ�̼���Ʒ�Ӧ����5.0g��������ô����10.0g�����������Ȼ�����Һ������Ϊ111.0g�����Ծݴ���ͼΪ��

��3����ַ�Ӧ���ձ������Ȼ��Ƶ��������������ǣ�
��100%=10%��
�ʴ�Ϊ����1����Ʒ��̼���Ƶ�������10.6g��2������ͼ����3����ַ�Ӧ���ձ������Ȼ��Ƶ���������������10%��
��1����Ҫ����10.0g̼�����Ҫ̼���Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy
Na2CO3+CaCl2�TCaCO3��+2NaCl
106 100 117
x 10.0g y
| 106 |
| x |
| 100 |
| 10.0g |
| 117 |
| y |
��2������ͼ����Ϣ����֪���Ƚ��ձ�����ձ����е����ݿ���֪����55.5g�Ȼ�����Һ���Ժ�̼���Ʒ�Ӧ����5.0g��������ô����10.0g�����������Ȼ�����Һ������Ϊ111.0g�����Ծݴ���ͼΪ��

��3����ַ�Ӧ���ձ������Ȼ��Ƶ��������������ǣ�
| 11.7g+(11g-10.6g) |
| 11g+120.0g-10g |
�ʴ�Ϊ����1����Ʒ��̼���Ƶ�������10.6g��2������ͼ����3����ַ�Ӧ���ձ������Ȼ��Ƶ���������������10%��
���������������Ŀʱ�����ȣ�Ҫ��Ǻ������йغ��������ʵĻ�ѧ��Ӧ���йؼ���ķ����������ʽ�����֪ʶ��Ȼ���������������龰��ͼ����Ϣ�ȣ������ѧ�����֪ʶ�ͼ��ܽ��з������㣮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
������������̼�ǡ����ߵĻ�ѧ���ʡ������µ�������Ҫ���壮�������ڼ��������Ͷ�����̼�������в���ѡ�õ��ǣ�������
| A��ȼ�ŵ�ľ�� |
| B����ɫ��̪��Һ |
| C����ɫʯ����Һ |
| D������ʯ��ˮ |
���������е������dz�ȥ�������õģ�������ȷ���ǣ�������
| A��������̼������������һ����̼�������������� |
| B�����������������������Ȼ��⣨����������������Һ�� |
| C���Ȼ�����Һ�������������ƣ������Ȼ�����Һ�� |
| D������������Һ��������̼���ƣ������Ȼ�����Һ�� |
 ��ͼΪA��B��C�������ʵ��ܽ�����ߣ���ͼ�ش�
��ͼΪA��B��C�������ʵ��ܽ�����ߣ���ͼ�ش�
