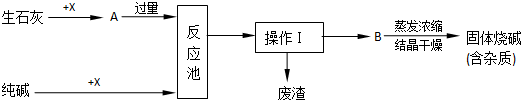
��Ŀ����
��������0.1mol/L NaOH��Һ500mL����ͼ��ijͬѧת����Һ��ʾ��ͼ��

��1����ͼ�еĴ����� ������ͼ�и����������ͷ�����ƽ�⣬Ϊ���ʵ�黹��Ҫ�������У� ��
�ڸ��ݼ����֪������NaOH������Ϊ g
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ� ��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ
B��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
C�����ܽ������������Һ�ز�����ע��500mL������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1-2cm��
��2������A�У���ϴ��Һ����������ƿ����Ŀ���� ����Һע������ƿǰ��ָ������£�������Ϊ ��
��3�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�ڳ��������г�ʱ������������ƹ��� ����û�н���A���� ����������ˮʱ���������˿̶��� ��������ʱ���ӿ̶��� ��δ���ܽ��������Һ�ָ������¾�ת����Һ ��ת����Һʱ��������Һ���� ��

��1����ͼ�еĴ�����
�ڸ��ݼ����֪������NaOH������Ϊ
������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ
B��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
C�����ܽ������������Һ�ز�����ע��500mL������ƿ��
D��������ƿ�ǽ�����ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1-2cm��
��2������A�У���ϴ��Һ����������ƿ����Ŀ����
��3�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
�ڳ��������г�ʱ������������ƹ���
���㣺һ������������������Һ������
ר�⣺��Һ����Һ���ܽ��
��������1����������һ������������������Һ�ķ����Ͳ�����Ѱ��װ���еĴ����ж�ʹ�������Ͳ������裻
��2��������ҺʱӦ����Һȫ����������ƿ����������Һ���ƫ��
��2��������ҺʱӦ����Һȫ����������ƿ����������Һ���ƫ��
����⣺��1�������Ʋ����м��㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȵȲ�����һ������ƽ��������ҩ��ȡ��ҩƷ�����ձ����ܽ⣬��ȴ��ת�Ƶ�500mL����ƿ�У����ò���������������ˮ��Һ�����̶���1��2cmʱ�����ý�ͷ�ιܵμӣ�������Ҫ������Ϊ����ƽ��ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
�ʴ�Ϊ��δ�ò�����������ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
������500mL0.1mol/LNaOH��Һ������n��NaOH��=0.1mol/L��0.5L=0.05mol��
m��NaOH��=0.05mol��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
���ɢ�֪��������Һ500mL������˳��ΪB��C��A��F��E��D��
�ʴ�Ϊ��B��C��A��F��E��D��
��2����֤����ȫ��ת������ƿ������ϴ��Һ����������ƿ����������Һ���ƫ����ȴ����ʱ��Һ�����С����Ũ�Ȼ���
�ʴ�Ϊ��������ȫ��ת������ƿ����С��������������Һ������ƿ���һ�£���С������
��3���������������ڿ������׳��⣬���������̼��Ӧ����̼������ˮ�����������ˣ������������������ƹ�������ƫ�ͣ�
���ձ��Ͳ�������մ�����ʣ�δϴ���ձ��Ͳ����������ʵ�����ƫ�٣�Ũ��ƫ�ͣ�
������ˮʱ���������˿̶ȣ���Һ�����ƫ��������Һ��Ũ��ƫ�ͣ�
����ʱ���ӿ̶��ߣ�Һ���ڿ̶����·�����Һ�����ƫ��������ҺŨ��ƫ�ߣ�
���������ܽ��û�лָ������£���ת�Ƶ�����ƿ�н������ƣ����ָ�������ʱ��Һ������С�����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
ת����Һʱ������������������ƿ���棬��Ϊ�����ƺõ���Һ��������Һ���о�һ�ԣ����������������������������������ı䣮
�ʴ��ǣ�ƫ�ͣ�ƫ�ͣ�ƫ�ͣ�ƫ�ߣ�ƫ�ߣ���Ӱ�죮
�ʴ�Ϊ��δ�ò�����������ҩ�ס��ձ�����������500mL����ƿ����ͷ�ιܣ�
������500mL0.1mol/LNaOH��Һ������n��NaOH��=0.1mol/L��0.5L=0.05mol��
m��NaOH��=0.05mol��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
���ɢ�֪��������Һ500mL������˳��ΪB��C��A��F��E��D��
�ʴ�Ϊ��B��C��A��F��E��D��
��2����֤����ȫ��ת������ƿ������ϴ��Һ����������ƿ����������Һ���ƫ����ȴ����ʱ��Һ�����С����Ũ�Ȼ���
�ʴ�Ϊ��������ȫ��ת������ƿ����С��������������Һ������ƿ���һ�£���С������
��3���������������ڿ������׳��⣬���������̼��Ӧ����̼������ˮ�����������ˣ������������������ƹ�������ƫ�ͣ�
���ձ��Ͳ�������մ�����ʣ�δϴ���ձ��Ͳ����������ʵ�����ƫ�٣�Ũ��ƫ�ͣ�
������ˮʱ���������˿̶ȣ���Һ�����ƫ��������Һ��Ũ��ƫ�ͣ�
����ʱ���ӿ̶��ߣ�Һ���ڿ̶����·�����Һ�����ƫ��������ҺŨ��ƫ�ߣ�
���������ܽ��û�лָ������£���ת�Ƶ�����ƿ�н������ƣ����ָ�������ʱ��Һ������С�����������Һ�����ƫС��������Һ��Ũ��ƫ�ߣ�
ת����Һʱ������������������ƿ���棬��Ϊ�����ƺõ���Һ��������Һ���о�һ�ԣ����������������������������������ı䣮
�ʴ��ǣ�ƫ�ͣ�ƫ�ͣ�ƫ�ͣ�ƫ�ߣ�ƫ�ߣ���Ӱ�죮
���������⿼����һ����������������Һ�����ƣ���ʵ���������Ŀ��ͬѧ����ʵ�������Ҫ�ܹ���������Ӧ�ã���Ŀ�ѶȲ���

��ϰ��ϵ�д�
 ��Ȥ����¹�֪��ϵ�д�
��Ȥ����¹�֪��ϵ�д� Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�
�����Ŀ
���и���������ʣ�ֻ��ˮ���ܼ�����ǣ�������
| A��NaCl��Na2CO3 |
| B��K2SO4��KCl |
| C��NH4NO3��NaOH |
| D��CuSO4��Fe2��SO4��3 |
 С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮
С��ͬѧΪ�˲ⶨ�������۵�ijƷ�ƴ������Ϊ�Ȼ��ƣ���̼���Ƶ���������������������ʵ�飺���������ձ��зֱ����11.0g��Ʒ�����������ձ��зֱ����һ��������10.0%�Ȼ�����Һ���۽���ַ�Ӧ�����ɵij������ˡ�ϴ�ӡ�����������õ��İ�ɫ���壮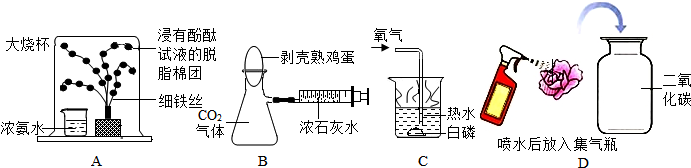
 С��ͬѧ��ѧϰ������ɷֲⶨʵ���Ľ��˿α��ϵ�װ��ͼ��ͼ���������ϵó����ͺ���һ���п�ȼ�ԣ���ȼ�պ��������Ҳ��ͬ����������40�漴��ȼ�գ��ش��������⣺
С��ͬѧ��ѧϰ������ɷֲⶨʵ���Ľ��˿α��ϵ�װ��ͼ��ͼ���������ϵó����ͺ���һ���п�ȼ�ԣ���ȼ�պ��������Ҳ��ͬ����������40�漴��ȼ�գ��ش��������⣺