��Ŀ����
���ⶨijʯ��ʯ��Ʒ��̼��Ƶ�����������̼����ڸ����·ֽ����������ƺͶ�����̼��ʯ��ʯ��Ʒ�е��������Ȳ��ֽ⣬�Ҳ����ᷴӦ�����ס�����λͬѧ�ֱ��������������ʵ�鷽����
����һ���ٳ�ȡʯ��ʯ��Ʒ����8g�����þƾ��Ƽ�����Ʒ��ֱ���������ٸı䣻�۽������������ڸ������������ȴ��Ƶ�����Ϊ6.9g���ܼ��㣮
���������ٳ�ȡʯ��ʯ��Ʒ����8g���ڼ�����������Ϊ7.3%������l00g��ʹ̼�����ȫ��Ӧ������Ӧ�����Һ�м��˺�����3.2g������������Һ��ǡ���кͶ��������ܼ��㣮
��ش������й����⣺
��1��̼����ڸ����·ֽ�Ļ�ѧ����ʽ�� ��
��2��100g��������Ϊ7.3%�������У������Ȼ��������Ϊ g��
��3���ɷ���һ���ṩ�����ݣ��ɼ���õ���Ʒ��̼��Ƶ���������Ϊ ��
��õ���������ƫС��ԭ�������
��4��������Ʒ��̼��Ƶ�����������
����һ���ٳ�ȡʯ��ʯ��Ʒ����8g�����þƾ��Ƽ�����Ʒ��ֱ���������ٸı䣻�۽������������ڸ������������ȴ��Ƶ�����Ϊ6.9g���ܼ��㣮
���������ٳ�ȡʯ��ʯ��Ʒ����8g���ڼ�����������Ϊ7.3%������l00g��ʹ̼�����ȫ��Ӧ������Ӧ�����Һ�м��˺�����3.2g������������Һ��ǡ���кͶ��������ܼ��㣮
��ش������й����⣺
��1��̼����ڸ����·ֽ�Ļ�ѧ����ʽ��
��2��100g��������Ϊ7.3%�������У������Ȼ��������Ϊ
��3���ɷ���һ���ṩ�����ݣ��ɼ���õ���Ʒ��̼��Ƶ���������Ϊ
��õ���������ƫС��ԭ�������
��4��������Ʒ��̼��Ƶ�����������
���㣺���ݻ�ѧ��Ӧ����ʽ�ļ���
ר�⣺�йػ�ѧ����ʽ�ļ���
��������1������̼��Ƹ����������������ƺͶ�����̼���н��
��2��������������=��Һ���������������������н��
��3�����ݷ���һ�м��ٵ��������Ƕ�����̼�����������ö�����̼�����������Ʒ��̼��Ƶ������������ɣ�
��4���������֪������һ������̼��Ʒ�Ӧ��һ�������������Ʒ�Ӧ�������������Ƶ���������������������Ʒ�Ӧ��������������������̼��Ʒ�Ӧ�����������������̼��ƺ����ᷴӦ�Ļ�ѧ����ʽ�����̼��Ƶ��������ٸ�������������ʽ���㼴�ɣ�
��2��������������=��Һ���������������������н��
��3�����ݷ���һ�м��ٵ��������Ƕ�����̼�����������ö�����̼�����������Ʒ��̼��Ƶ������������ɣ�
��4���������֪������һ������̼��Ʒ�Ӧ��һ�������������Ʒ�Ӧ�������������Ƶ���������������������Ʒ�Ӧ��������������������̼��Ʒ�Ӧ�����������������̼��ƺ����ᷴӦ�Ļ�ѧ����ʽ�����̼��Ƶ��������ٸ�������������ʽ���㼴�ɣ�
����⣺��1��̼��Ƹ����������������ƺͶ�����̼����ѧ����ʽ��CaCO3
CaO+CO2�������CaCO3
CaO+CO2����
��2��100g��������Ϊ7.3%�������У������Ȼ��������Ϊ=100g��7.3%=7.3g�����7.3��
��3��������̼������=8g-6.9g=1.1g
����Ʒ��̼��Ƶ�����Ϊx��
CaCO3
CaO+CO2��
100 44
x 1.1g
=
x=2.5g
��Ʒ��̼��Ƶ���������=
��100%=31.25%
�ƾ��Ʋ����ṩ̼��Ʒֽ�����Ҫ���¶ȣ���Ϊ̼���ֻ���ڸ��������²��ܹ��ֽ����������ƺͶ�����̼������̼��ƿ���û����ȫ�ֽ⣬�ʲ�õ���������ƫС�����31.25%���ƾ��Ʋ����ṩ̼��Ʒֽ�����Ҫ���¶ȣ�̼��ƿ���û����ȫ�ֽ⣻
��4�������������Ʒ�Ӧ��HCl����Ϊz
HCl+NaOH�TNaCl+H2O
36.5 40
z 3.2g
=
��֮�ã�z=2.92g
��̼��Ʒ�Ӧ����������������Ϊ��7.3%��100g-2.92g=4.38g
��ʯ��ʯ��̼��Ƶ�����Ϊy
CaCO3+2HCl=CaCl2+CO2��+H2O
100 73
y 4.38g
=
��֮�� y=6g
̼��Ƶ���������Ϊ��
��100%=75%��
����Ʒ��̼��Ƶ���������Ϊ75%��
| ||
| ||
��2��100g��������Ϊ7.3%�������У������Ȼ��������Ϊ=100g��7.3%=7.3g�����7.3��
��3��������̼������=8g-6.9g=1.1g
����Ʒ��̼��Ƶ�����Ϊx��
CaCO3
| ||
100 44
x 1.1g
| 100 |
| x |
| 44 |
| 1.1g |
x=2.5g
��Ʒ��̼��Ƶ���������=
| 2.5g |
| 8g |
�ƾ��Ʋ����ṩ̼��Ʒֽ�����Ҫ���¶ȣ���Ϊ̼���ֻ���ڸ��������²��ܹ��ֽ����������ƺͶ�����̼������̼��ƿ���û����ȫ�ֽ⣬�ʲ�õ���������ƫС�����31.25%���ƾ��Ʋ����ṩ̼��Ʒֽ�����Ҫ���¶ȣ�̼��ƿ���û����ȫ�ֽ⣻
��4�������������Ʒ�Ӧ��HCl����Ϊz
HCl+NaOH�TNaCl+H2O
36.5 40
z 3.2g
| 36.5 |
| z |
| 40 |
| 3.2g |
��֮�ã�z=2.92g
��̼��Ʒ�Ӧ����������������Ϊ��7.3%��100g-2.92g=4.38g
��ʯ��ʯ��̼��Ƶ�����Ϊy
CaCO3+2HCl=CaCl2+CO2��+H2O
100 73
y 4.38g
| 100 |
| y |
| 73 |
| 4.38g |
��֮�� y=6g
̼��Ƶ���������Ϊ��
| 6g |
| 8g |
����Ʒ��̼��Ƶ���������Ϊ75%��
������������Ҫ����ѧ�����ü��跨�ͻ�ѧ����ʽ���м�����ƶϵ�����������ʱҪע��淶�Ժ�ȷ�ԣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
���л�ѧʵ�����Ҫ����������ȷ���ǣ�������
| A��������ԭ������--��ɫ�����ɺ�ɫ |
| B������������ȼ��--��������ɫ���� |
| C��ϸ��˿�ڿ�����ȼ��--�������䣬���ɺ�ɫ���� |
| D�������ڿ�����ȼ�ղ��������İ��� |
��ͼʵ�������ȷ���ǣ�������
A��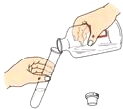 �㵹Һ�� |
B�� ��������ζ |
C��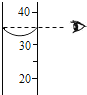 ��Һ����� |
D��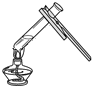 ��Һ����� |
�����ܷ������ֽⷴӦ���ǣ�������
| A������ϡ���� |
| B������ͭ���������� |
| C��������Ȼ��� |
| D��������������� |
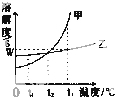 ��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�
��ͼ�Ǽס������ֹ������ʵ��ܽ�����ߣ�