��Ŀ����
20����ͼ��ʾijЩ���ʼ�ת����ϵ����������ʾ����֮�����ת����ϵ��������A��B������ͬԪ����ɵ���ɫҺ�壬��A��������ɱ�����ã�C��FΪ���嵥�ʣ�F�ǿ����к�����ߵ����壻D��EΪ���壬D�ʺ���ɫ��E��Ӧ����㷺�Ľ�����G����Է�������Ϊ100�����Ԫ�ص�ԭ�Ӹ�����Ϊ3��2���ش��������⣺

��1������D�������Ǵ����ã�
��2���ڴ�����A��ͬ������ţ�A���е� B���ܶ� C���ܽ��ԣ���������������壻
��3����Ӧ�ۢܵĻ�ѧ����ʽ����3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2����Mg3N2+6H2O=3Mg��OH��2��+2NH3����
��4��M��NH3����������[CO��NH2��2]����μӷ�Ӧ��M��NH3��������Ϊ22��17��
���� ����A��B������ͬԪ����ɵ���ɫҺ�壬��A��������ɱ�����ã�C��FΪ���嵥�ʣ�A�ֽ������B��C������A�ǹ���������Һ��B��ˮ��C��������D�Ƕ������̣�F�ǿ����к�����ߵ����壬����F�ǵ�����E��Ӧ��
��㷺�Ľ���������E����������������ˮ��һ�����������⣬����D������������������һ����̼�����������Ͷ�����̼������M�Ƕ�����̼��������þ�ڵ�ȼ�����������ɵ���þ������G�ǵ���þ������þ��ˮ��Ӧ����������þ�Ͱ�����Ȼ���Ƴ������ʽ�����֤���ɣ�
��� �⣺��1��A��B������ͬԪ����ɵ���ɫҺ�壬��A��������ɱ�����ã�C��FΪ���嵥�ʣ�A�ֽ������B��C������A�ǹ���������Һ��B��ˮ��C��������D�Ƕ������̣�F�ǿ����к�����ߵ����壬����F�ǵ�����E��Ӧ����㷺�Ľ���������E����������������ˮ��һ�����������⣬����D������������������һ����̼�����������Ͷ�����̼������M�Ƕ�����̼��������þ�ڵ�ȼ�����������ɵ���þ������G�ǵ���þ������þ��ˮ��Ӧ����������þ�Ͱ�����������֤���Ƶ���ȷ�����Ԣ��ж������̵������Ǵ����ã�
��2���ڴ�����Һ̬�����õ������������������÷е㲻ͬ����ѡ��A��
��3������һ����̼���������ڸ��µ��������������Ͷ�����̼����ѧ����ʽΪ��3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2�����ǵ���þ��ˮ��Ӧ����������þ�����Ͱ�������ѧ����ʽΪ��Mg3N2+6H2O=3Mg��OH��2��+2NH3����
��4��������̼��NH3����������[CO��NH2��2]����ѧ����ʽΪ��CO2+2NH3$\frac{\underline{\;����\;}}{��}$CO��NH2��2+H2O����μӷ�Ӧ�Ķ�����̼��NH3��������Ϊ22��17��
�ʴ�Ϊ����1�������ã�
��2��A��
��3����3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2����Mg3N2+6H2O=3Mg��OH��2��+2NH3����
��4��22��17��
���� �ڽ������ʱ�����Ƚ������������������Ƴ���Ȼ�����Ƴ������ʺ����е�ת����ϵ�Ƶ�ʣ������ʣ�����Ƴ��ĸ������ʴ���ת����ϵ�н�����֤���ɣ�

| A�� | ���� | B�� | ���� | C�� | ���� | D�� | �ɱ� |
| A�� | �ü�ȩ��Һ���ݺ���Ʒ | B�� | �ù�ҵ�������������� | ||
| C�� | ţ�̾����ͺ�õ����� | D�� | ù����ʵĴ��ס����� |
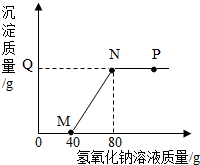 ��100.0g���������ͭ�Ļ����Һ����μ���10.0%������������Һ�����������������������������Һ��������ϵ��ͼ��ʾ������˵����ȷ���ǣ�������
��100.0g���������ͭ�Ļ����Һ����μ���10.0%������������Һ�����������������������������Һ��������ϵ��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | P����Һ�е�������2�֣���Һ��pH=7 | |
| B�� | �ӵ�M����Nʱ����Һ����������40g��Q�����ֵΪ4.9 | |
| C�� | ��Һ��Na2SO4����������������N��P | |
| D�� | ԭ�����Һ�������������������Ϊ9.8% |
| A�� | ������������������ƺ�ʳ�� | |
| B�� | �������ƿ��Լӵ�ʳƷ�У���������Ҫ���Ϲ涨 | |
| C�� | �����������ȷֽ�ų������������NH3 | |
| D�� | ��������������Ԫ����� |
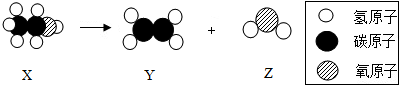
| A�� | X��Y��Z������� | |
| B�� | �÷�Ӧ˵�������ڻ�ѧ�仯�в��ɷ� | |
| C�� | X��̼����Ԫ�ص���������1��3 | |
| D�� | X�Ļ�ѧʽ��C2H5OH |
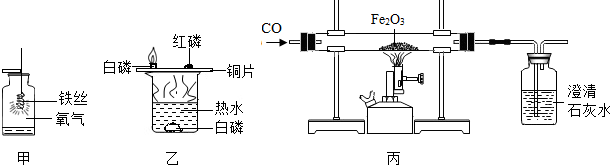
 �ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㣮
�ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㣮