��Ŀ����
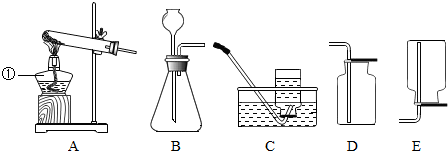
2��ʵ������ȡ����ͨ��������ˮ�����ƺͼ�ʯ�ҹ������ﹲ�ȵķ�����������ܶȱȿ����ܶ�С��������ˮ����1��Aװ��ͼ�У������ٵ������Ǿƾ��ƣ�
��2��ʵ������ȡ���ռ����������������AC����AE����ѡ������װ��ͼ��ţ���
��3����ͼ������������������Ϊ10%��NaCl��Һ��ʵ�����ʾ��ͼ��
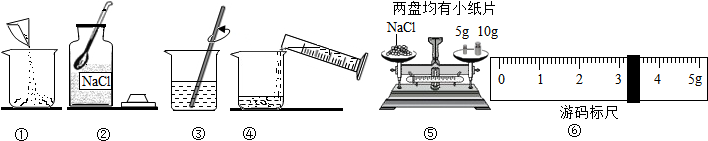
A����ͼ��ʾ����ű�ʾ������Һ����ȷ����˳��
B������NaClʱ����ƽƽ����״̬��ͼ����ʾ��������ʾ����ͼ�����ȡ��NaCl����Ϊ18.2g��
C������NaCl������ϷŻ�����ʱ��������һ������ȱ����һ��С�ǣ�����������������ȷ����������Һ��������������С�ڣ�����ڡ�����С�ڡ����ڡ���10%��
���� ��1���ݳ��������ش�
��2���ݷ�Ӧ��״̬ѡ����װ�ã��������ܶȺ��ܽ���ѡ���ռ�װ�ã�
��3��ʹ����ƽ��ȡ�Ȼ��Ƶ�����ʱ��Ҫ��������������̣�����������̣������������=����+���룻
��4��������������������ʽ���������Һ���������Ӷ����ˮ���������ٸ����ܶȹ�ʽ����ˮ�����������ȱ��һ���������ʳ�ε�������С�����Ծݴ˽����⣮
��� �⣺��1������������������ȵľƾ��ƣ�
��2������ˮ�����ƺͼ�ʯ�ҹ������ﹲ�ȵķ�����ȡ���飬���ڹ�������ͣ���ѡ����װ��A��������ܶȱȿ����ܶ�С��������ˮ�����Կ��������ſ���������ˮ���ռ���
��3��ʳ�ε�����=����+���룬��ͼ��֪������Ķ�����15g������Ķ�����3.2g����ʳ�ε�����=15g+3.2g=18.2g��
��4������ȱ��һ���������ʳ�ε�������С�����ʼ��٣�����Һ��ϡ����������������С��
�ʴ�Ϊ����1���ƾ��ƣ�
��2��AC����AE����
��3��18.2g��
��4��С�ڣ�
���� ���⿼������ȡ����ij���װ�á��������ռ�װ�õ�ѡȡ����Һ�����ƣ���ɴ��⣬�����������е�֪ʶ��ϲ���������������ʽ������𣬷���װ�þݷ�Ӧ��״̬�ͷ�Ӧ����ѡ���ռ�װ�þ������ܶȺ��ܽ���ѡ��

 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�| A�� | �ռ������������θ����� | |
| B�� | �����������̼������ƿ�м���Լ$\frac{1}{3}$�����ˮ���Ǻ�ƿ�Ǻ���ƿ�ӱ�� | |
| C�� | �̬�������ľ�һ��ʹ�ÿ���������߷�Ч | |
| D�� | ��������������ͭ��Һ�У��������к�ɫ���帽�ţ���Һ���������� |
| A�� | O2 | B�� | 2H | C�� | F e | D�� | H2O |
| A�� | NH4+NO3-Na+ Cl- | B�� | CO32- K+ Na+ SO42- | ||
| C�� | Cu2+ OH- Cl- Ba2+ | D�� | Ag+NO3- Cl- Na+ |
| A�� | 87a��158b | B�� | 172a��90b | C�� | 158a��87b | D�� | 90a��172b |
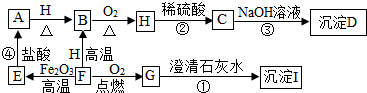 ���п�ͼ�е����ʶ��dz��н�ѧ���ģ�����E��F��H��Ϊ��ɫ��ĩ��BΪ�Ϻ�ɫ���壬DΪ��ɫ����������֮������ͼ��ת����ϵ����������������ʡȥ��
���п�ͼ�е����ʶ��dz��н�ѧ���ģ�����E��F��H��Ϊ��ɫ��ĩ��BΪ�Ϻ�ɫ���壬DΪ��ɫ����������֮������ͼ��ת����ϵ����������������ʡȥ�� ����[CO��NH2��2]��һ�ֵ��ʣ���������ҺҲ��������������β������Һ����ͼΪij��˾�����ij���������Һ�ı�ǩ����������Һ������������β������NO��ʱ�Ļ�ѧ����ʽ���£���Ӧ������ȥ����2CO��NH2��2+4NO+O2=2CO2+4N2+4H20
����[CO��NH2��2]��һ�ֵ��ʣ���������ҺҲ��������������β������Һ����ͼΪij��˾�����ij���������Һ�ı�ǩ����������Һ������������β������NO��ʱ�Ļ�ѧ����ʽ���£���Ӧ������ȥ����2CO��NH2��2+4NO+O2=2CO2+4N2+4H20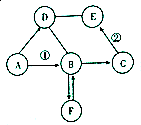 ��֪A��B��C��D��E��F�dz��л�ѧ�г������������ʣ�����AΪ-һ�ֳ������ᣬB��C��DΪ�����EΪ�����ҷ�ĩΪ��ɫ��F�����ڽ������ϣ�����֮����һ������������ͼ��ʾ��ת����ϵ��ͼ�С�������ʾ���ʼ����ת����ϵ����һ����ʾ�������ʼ��ܷ�����Ӧ�����ַ�Ӧ�������������ȥ�����밴Ҫ��ش��������⣺
��֪A��B��C��D��E��F�dz��л�ѧ�г������������ʣ�����AΪ-һ�ֳ������ᣬB��C��DΪ�����EΪ�����ҷ�ĩΪ��ɫ��F�����ڽ������ϣ�����֮����һ������������ͼ��ʾ��ת����ϵ��ͼ�С�������ʾ���ʼ����ת����ϵ����һ����ʾ�������ʼ��ܷ�����Ӧ�����ַ�Ӧ�������������ȥ�����밴Ҫ��ش��������⣺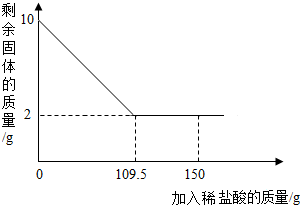 ��һ��������Ʒ����Ҫ�ɷ�Ϊ����������ijѧУ��ѧ��ȤС��Ϊ�˲����Ʒ������������������������������̽����С��ȡ10g��������Ʒ�����ʲ�����ˮ���ᣩ�����ϼ���ϡ���ᵽ������������ټ��٣�����ϡ���������ͼ��ʾ����
��һ��������Ʒ����Ҫ�ɷ�Ϊ����������ijѧУ��ѧ��ȤС��Ϊ�˲����Ʒ������������������������������̽����С��ȡ10g��������Ʒ�����ʲ�����ˮ���ᣩ�����ϼ���ϡ���ᵽ������������ټ��٣�����ϡ���������ͼ��ʾ����