��Ŀ����
19��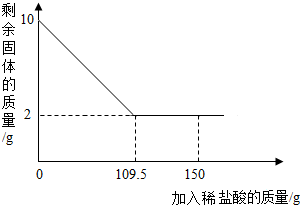 ��һ��������Ʒ����Ҫ�ɷ�Ϊ����������ijѧУ��ѧ��ȤС��Ϊ�˲����Ʒ������������������������������̽����С��ȡ10g��������Ʒ�����ʲ�����ˮ���ᣩ�����ϼ���ϡ���ᵽ������������ټ��٣�����ϡ���������ͼ��ʾ����
��һ��������Ʒ����Ҫ�ɷ�Ϊ����������ijѧУ��ѧ��ȤС��Ϊ�˲����Ʒ������������������������������̽����С��ȡ10g��������Ʒ�����ʲ�����ˮ���ᣩ�����ϼ���ϡ���ᵽ������������ټ��٣�����ϡ���������ͼ��ʾ������1����ʹ�õ�ϡ�����������������
��2����ϡ����ͳ�����ǡ����ȫ��Ӧʱ��������Һ�������ǣ����������������������������ǣ���
��3���������ۣ�С��Ҳȡ��10�˳�������Ʒ���Թ��У�ͨ��һ����̼һ��ʱ�����ȣ�����Թ��ڲ�������������8.2g��С����С������õĽ������������������������С���ģ�ƫС����ƫ��ƫС����Ӱ�죩��ԭ������ǣ�����дһ�ּ��ɣ�
���� �����������������Ӧ�����Ȼ�����ˮ�����ݼ���������������м���μӷ�Ӧ��������������������������������Ȼ������������з�����
��� �⣺��1���������������ᷴӦ�����ʲ�����ˮ���ᣬ���Բμӷ�Ӧ��������������Ϊ��10g-2g=8g������������������Ϊ��$\frac{8g}{10g}$��100%=80%��
��μӷ�Ӧ�����������Ϊx
Fe2O3+6HCl=2FeCl3+3H2O
160 219
8g x
$\frac{160}{8g}$=$\frac{219}{x}$
x=10.95g
������ʹ�õ�ϡ�����������������Ϊ��$\frac{10.95g}{109.5g}$��100%=10%��
��ϡ����ͳ�����ǡ����ȫ��Ӧʱ��������Һ�������ǣ�109.5g+8g=117.5g��
��3��
Fe2O3+6HCl=2FeCl3+3H2O �������
160 219 48
y 1.8g
$\frac{160}{y}$=$\frac{48}{1.8g}$
y=6g
$\frac{6g}{10g}$��100%=60%���Թ��ڲ��������������������ʵ�������
�ʴ�Ϊ����1��10%��
��2��117.5g��80%��
��3��ƫС���Թ��ڲ��������������������ʵ�������
���� ��ͬ��Ҫ�����˻�ѧ����ʽ�ļ��㣬�ѶȲ���ע�����Ĺ淶�Ժ�ȷ�ԣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | �����κ�ˮ�ķ�Ӧ�����кͷ�Ӧ�����ռ���Ը����������� | |
| B�� | ����������������θ����ࣻ�ɵ�ؿ��Խ�����ת��Ϊ��ѧ�� | |
| C�� | ά���ز����ṩ�������Զ������ô�������������������Ԫ�أ�Ӧ�������� | |
| D�� | ʹ������Դ���ܼ��������������ʳ�ؽ����Σ����������õ����ţ�̽ⶾ |
| A�� |  ����ҺpH | B�� |  ���� | C�� |  ϡ��Ũ���� | D�� |  ��������� ��������� |

| A�� | ����һ�������� | B�� | �ڢ�����ͬ��Ԫ�� | C�� | �ܴ�һ������� | D�� | �ڻ�ѧ���ʲ����� |
| ѡ�� | ���� | ���ʣ������� | �Լ����������� |
| A | ������̼ | ˮ���� | ͨ���������������ƹ������� |
| B | ϡ���� | ϡ���� | �����������������Һ������ |
| C | �Ȼ��� | ��ɳ | ����������ˮ���ܽ⡢�������ᾧ |
| D | ͭ�� | ���� | ���������ϡ���ᣬ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |