��Ŀ����
13�����ж�Ӧ�Ļ�ѧ����ʽ��д��ȫ��ȷ���ǣ�������| A�� | ��˿��������ȼ�գ�2Fe+3O2 $\frac{\underline{\;��ȼ\;}}{\;}$ 2Fe2O3 | |
| B�� | һ����̼��ԭ��������3CO+Fe2O3=2Fe+3CO2 | |
| C�� | ���ܱ�������ȼ�պ�����֤�����غ㶨�ɣ�2P+O2 $\frac{\underline{\;��ȼ\;}}{\;}$ P2O5 | |
| D�� | �ó����ʯ��ˮ���������̼���壺CO2+Ca��OH��2=CaCO3��+H2O |
���� ���ݻ�ѧ����ʽ�ж�����ķ����迼�ǣ�Ӧ�õ�ԭ���Ƿ���ȷ����ѧʽ��д�Ƿ���ȷ���Ƿ���ƽ����Ӧ�����Ƿ���ȷ�����͡��ı�ע�Ƿ���ȷ��
��� �⣺A����ѧ����ʽ�����Ͽ���ʵ�������ﻯѧʽд����Ӧ��Fe3O4����ȷ��ѧ����ʽΪ��3Fe+2O2$\frac{\underline{\;��ȼ\;}}{\;}$Fe3O4������
B��ȱ�ٷ�Ӧ����������ȷ�Ļ�ѧ����ʽΪ��3CO+Fe2O3$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2������
C����ѧ����ʽû����ƽ����ȷ��ѧ����ʽΪ��4P+5O2 $\frac{\underline{\;��ȼ\;}}{\;}$ 2P2O5������
D���û�ѧ����ʽ��д��ȫ��ȷ��
��ѡD
���� ������Ҫ���黯ѧ����ʽ����д���������ɣ�����Ŀ�е���Ϣ����֪��Ӧ��������Ӧ��������д�˻�ѧ����ʽ�ķ�����

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
1����FeO��Fe2O3��CaCO3����������ɵĻ�����У���֪��Ԫ�ص���������Ϊ56%�����ڸû�����и�Ԫ�ص��������������ǣ�������
| A�� | 35% | B�� | 25% | C�� | 20% | D�� | 10% |
8��20��ʱ��NaCl�ܽ���ˮ��ʵ���������±���������������ȷ���ǣ�������
| ʵ����� | �� | �� | �� | �� |
| ˮ��������g�� | 10 | 10 | 10 | 10 |
| ����NaCl��������g�� | 2 | 3 | 4 | 5 |
| ��Һ��������g�� | 12 | 13 | 13.6 | 13.6 |
| A�� | ����������Һ�DZ�����Һ | B�� | 20��ʱ10gˮ������ܽ�4gNaCl | ||
| C�� | �ۢ���Һ��Ũ����ͬ | D�� | ����Һ�Dz�������Һ |
18��ͼl��ʾ��һ�����Ȼ��ƺ�ϡ����Ļ����Һ�еμ�̼������Һ���������ʾ�����̼������Һ���������������ʾʵ���еõ��ij����������������ͼ2��ʾһ�����������X�Ͳ�ͬ�����������Ӧ������Ӧ����ˮ��ˮΪҺ̬�����������ʾͨ�������������������ʾ��Ӧ����������������Ӧǰ������¶���ѹǿ��ͬ��ͬ��ͬѹ�£���ͬ������κ����庬����ͬ�ķ���������������������ȷ���ǣ�������
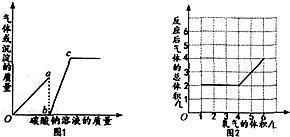

| A�� | ͼl��oa�α�ʾʵ���г������õı仯��� | |
| B�� | ͼl��c���ʾ���������Һ��̼������Һǡ����ȫ��Ӧ | |
| C�� | ��ͼ2��֪����ӦǰX�����Ϊ2L | |
| D�� | ��ͼ2��֪��x������C0��CH4 |
5��������ɽ��Ĺ������ͭ������һЩ����ɫ���ʣ��������׳ơ�ͭ�⡱���仯ѧ���ΪaCuCO3•bCu��OH��2•cH2O��a��b��cΪ��������ȣ���С��ͬѧΪ�ⶨ����ɣ���ȡ����ɫ����25.8g��������ʵ�飺
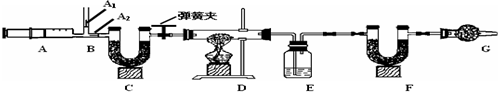
��֪����CuCO3$\frac{\underline{\;\;��\;\;}}{\;}$CuO+CO2������Cu��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CuO+H2O
��ͼ��B��Ϊ����������ע����ʱA1�رգ�A2������ע����ʱ��A1��������A2�رգ�
��һ��ʵ�鲽�� ����װ��װ�ã���������Ԣڷ�������ע�����۳���E��F�������ܹرյ��ɼУ�����D���Թ�ֱ����Ӧ���ٽ��Тݴ��ɼУ��ٴη�����������ע�������ٴγ���E��F��������
����������̽����
��1��E�е�ҩƷΪŨ���ᣬE������������ˮ������C��F��G��װ�м�ʯ�ң�CaO��NaOH�Ĺ����������C�������dz�ȥ�����еĶ�����̼��F���������������ɵĶ�����̼��G�������Ƿ�ֹ�����Ķ�����̼��ˮ��������Fװ�ã�
��2��ʵ�鲽�������ܷ�ߵ����ܣ���ܡ����ܡ������������в���ݵIJ�����������õ�̼��ͭ��������ƫС���ƫ����ƫС��������Ӱ�족�����ò�������ע����ʱ������Ŀ����ʹ���ɵĶ�����̼��ˮ�������ճ�֣�

��֪����CuCO3$\frac{\underline{\;\;��\;\;}}{\;}$CuO+CO2������Cu��OH��2 $\frac{\underline{\;\;��\;\;}}{\;}$CuO+H2O
��ͼ��B��Ϊ����������ע����ʱA1�رգ�A2������ע����ʱ��A1��������A2�رգ�
��һ��ʵ�鲽�� ����װ��װ�ã���������Ԣڷ�������ע�����۳���E��F�������ܹرյ��ɼУ�����D���Թ�ֱ����Ӧ���ٽ��Тݴ��ɼУ��ٴη�����������ע�������ٴγ���E��F��������
����������̽����
| ��Ӧǰ | ��Ӧ�� |
| E������Ϊ100.0g | E������Ϊ105.4g |
| F������Ϊ50.0g | F������Ϊ54.4g |
��2��ʵ�鲽�������ܷ�ߵ����ܣ���ܡ����ܡ������������в���ݵIJ�����������õ�̼��ͭ��������ƫС���ƫ����ƫС��������Ӱ�족�����ò�������ע����ʱ������Ŀ����ʹ���ɵĶ�����̼��ˮ�������ճ�֣�
2��ijѧУ��ѧϰС��Ե��ص�ʯ��ʯ�������е��飬�ⶨʯ��ʯ��̼��Ƶ��������������õķ������£�ȡ��ʯ��ʯ��Ʒ16�˷����ձ�����80��ϡ����ƽ���ֳ��ķ����μ��룬���������������ݼ��±�����֪ʯ��ʯ��Ʒ�к��еĶ�����������ʲ�����ˮ��Ҳ����ϡ���ᷴӦ��������㣺
��1���ϱ���n����ֵΪ2.8��
��2����Ʒ��̼��Ƶ���������Ϊ82.5%��
��3������ͼ��ʾ���μ������������������������֮��Ĺ�ϵ�����������ʾ�μ�������������������ʾ���������������
��4��С��ͬѧ�������������������������������������£�
�⣺�������������������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73
13.2g 80x
100��73=13.2��80x
x=12%
С���ļ�������д������Դ�������ͨ����ʽ������о�����
| ��� | ����ϡ���������/�� | ʣ����������/�� |
| ��һ�� | 20 | 11 |
| �ڶ��� | 20 | 6 |
| ������ | 20 | 2.8 |
| ���Ĵ� | 20 | n |
��2����Ʒ��̼��Ƶ���������Ϊ82.5%��
��3������ͼ��ʾ���μ������������������������֮��Ĺ�ϵ�����������ʾ�μ�������������������ʾ���������������
��4��С��ͬѧ�������������������������������������£�
�⣺�������������������Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 73
13.2g 80x
100��73=13.2��80x
x=12%
С���ļ�������д������Դ�������ͨ����ʽ������о�����
3����ͼ�ǡ�β����ת������������β�����ж�����ת��Ϊ���������ʾ��ͼ�����в�ͬ��ԲȦ������ͬ��ԭ�ӣ�����˵����ȷ���ǣ�������
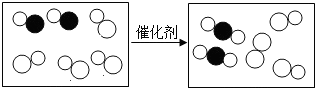

| A�� | �˷�Ӧ�е������� | |
| B�� | ԭ���ڻ�ѧ�仯���ǿɷֵ� | |
| C�� | �˷�Ӧ���������������� | |
| D�� | �μӷ�Ӧ�����ַ��ӵĸ�����Ϊ2��1 |