��Ŀ����
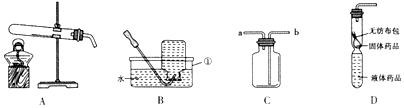
13����ͼ��ʵ���ҳ�����һЩװ�ã��������ѧ֪ʶ�ش��������⣺
��1�������ٵ���������ƿ��
��2��Ϊ�õ�ƽ�ȵ������������ռ��������������Ӧѡ��ķ���װ�ú��ռ�װ����B��D��д���йط�Ӧ�Ļ�ѧ����2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��3�����װ��A�������Եķ����ǣ��ɽ���ͼ�е�����װ�ã��Ȱѵ��ܵ�һ�˷���ˮ�У�Ȼ�����ֽ����Թܵ���ڣ��۲쵼�ܿ��Ƿ�������ð������װ���Թܿ��Գ�����б��ԭ���Ƿ�ֹ����ˮ������ը���Թܣ�
���� ��1��������������ƣ�
��2������װ��B���ص�ȷ����ȡװ�ã������ſ������ռ�������Ƚϸ����Լ���ѧ����ʽ��д����������
��3������װ�������Եļ��鷽���Լ���������ȵ�ע��������������
��� �⣺��1������������ƿ�������ƿ��
��2��ͨ��װ��B�е�ע�����μ�Һ���Լ������ʿ��Կ��ƻ�ѧ��Ӧ�Ŀ������������ܶȱȿ��������Կ��������ſ��������ռ��Ƚϸ��������������װ��B��ȡ��������Ϊ���������ڶ������̵Ĵ������·ֽ�Ϊˮ�����������B��D��2H2O2$\frac{\underline{\;MnO_2\;}}{\;}$2H2O+O2����
��3������װ�õ������Եķ����ǣ��Ȱѵ��ܵ�һ�˷���ˮ�У�Ȼ�����ֽ����Թܵ���ڣ��۲쵼�ܿ��Ƿ�������ð�������Թ��еĹ������ʱ��Ϊ�˷�ֹ����ˮ����ը���Թܣ������Թܿ�Ҫ��������б���ʴ�Ϊ���Ȱѵ��ܵ�һ�˷���ˮ�У�Ȼ�����ֽ����Թܵ���ڣ��۲쵼�ܿ��Ƿ�������ð������ֹ����ˮ������ը���Թܣ�
���� ��������Ҫ���������������ơ��������ȡװ�ú��ռ�װ�õ�ѡ��ͬʱҲ���������ֱ���ʽ����д��ע������ȣ��ۺ��ԱȽ�ǿ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����йأ����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�

 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�| A�� | ľ�Ȼ�����Һ���������Ժ�ɫ�ǻ�ѧ�仯 | |
| B�� | ��ʹľ�Ȼ�����Һ����ɫ��һ���Ǽ� | |
| C�� | ���Ȼ�����Һ����ľ�Ȼ�����Һ����Һ��Ϊ��ɫ | |
| D�� | ľ�Ȼ�����Һ���������ָʾ�� |
| ��ˮ�ܶ� | CaSO4 | NaCl | MgCl2 | MgSO4 | NaBr |
| 1.13 | 0.56 | ||||
| 1.20 | 0.91 | ||||
| 1.21 | 0.05 | 3.26 | 0.004 | 0.008 | |
| 1.22 | 0.015 | 9.65 | 0.01 | 0.04 | |
| 1.26 | 0.01 | 2.64 | 0.02 | 0.02 | 0.04 |
| 1.31 | 1.40 | 0.54 | 0.03 | 0.06 |
�ٺ�ˮ���ܶ�Ϊ1.21g/mlŨ����1.22g/mlʱ����˵����ȷ����C
A�������ε��ܽ������ B����ˮ��NaBr��������������
C������������NaCl�������������� D����ˮ��ˮ��������������
�ں�ˮ��Ũ�������У�������������������ƣ�
��2����ҵ�����ô���ˮ����������CaCl2��MgCl2�������������������ͼ��

��֪���������£�NH3��������ˮ��CO2������ˮ��
��NaHCO3�����ֽ⣬Na2CO3���Ȳ��ֽ⣮
���������գ�
�ٴ���ˮ���������NaOH��Na2CO3�ֱ��ȥMgCl2��CaCl2������CΪMg��OH��2��CaCO3��
�������У��ȡ���������̼�ữ����Ŀ���ǰ���ˮ�Լ��ԣ����������ն�����̼��
�ۡ�̼�ữ������˻�õ�NH4Cl���������ʣ�Ҳ���ȼ��ȣ�����ȡ�����ȴ����NH4Cl��Һ���ټ���ʯ��ˮ��ѭ��ʹ�õ������ǰ�����
�ܡ����ա�ʱ��NaHCO3�ֽ����ɵ�����D�Ƕ�����̼����������Dֱ����������������������ǵ�������ЧӦ��
��3�������о����Ժ���CaCl2��MgCl2��±ˮ�ͱ��ǣ���Ҫ�ɷ�ΪCaCO3��Ϊ��Ҫԭ����ʵ�����Ʊ���ˮCaCl2��������ͼ1��

��ش��������⣺
�ٲ��������õ��IJ����������ձ�����������©����
����MgCl2Ϊ��д��±ˮ�г�ȥMg2+ʱ������Ӧ�Ļ�ѧ����ʽCa��OH��2+MgCl2=Mg��OH��2��+CaCl2��
�۲������ữʱӦѡ�������HCl����д��ѧʽ����
��������Ӧ��δ�漰�Ļ�����Ӧ������b����д��ĸ����
a�����ֽⷴӦ b���û���Ӧ c�����Ϸ�Ӧ d���ֽⷴӦ
�ݻ��յ�CO2������������ʹ�������������ˮ������Ӧ�����ɼ�����������÷�Ӧ�Ļ�ѧ����ʽΪCO2+2H2O$\frac{\underline{\;����\;}}{������}$CH4+2O2������������ʱ���ڲ�ͬ�������١��ڡ��ۣ��������£�������������ʱ��ı仯��ͼ2��ʾ���ڵ�10Сʱʱ�����������Ǣڣ���д���١������ڡ����ۡ�����
| A�� | ����������ȼ�ղ�������ɫ���� | |
| B�� | ���ˮʱ���������缫�ϲ�������������2��1 | |
| C�� | ��һƿŨ���ᣬƿ�ڻ���ְ�ɫ���� | |
| D�� | þ�ڿ�����ȼ�շ���ҫ�۰� |
 ij��ѧ��ȤС��ͬѧ�������ͼ��ʾ��װ�ã��������ѧ֪ʶ�ش��й����⣺
ij��ѧ��ȤС��ͬѧ�������ͼ��ʾ��װ�ã��������ѧ֪ʶ�ش��й����⣺


 ʵ������һ������������غͶ���������ȡ�������ش��������⣮
ʵ������һ������������غͶ���������ȡ�������ش��������⣮