��Ŀ����
17���ò�ͬ��ӫ��Ⱦ�ϱ�ǵĿ��壬�ֱ�����ϸ����С��ϸ����ϸ��Ĥ�ϵ�һ�ֿ�ԭ���ʽ�ϣ�ʹ����ϸ���ֱ������ɫ�ͺ�ɫӫ�⣮��������ϸ���ںϳ�һ��ϸ��ʱ����ʼһ�����ɫ��һ��ʺ�ɫ������37���±���0.5h��������ɫ��ӫ���ͳʾ��ȷֲ�����ͼ�������ͼ�ش��������⣺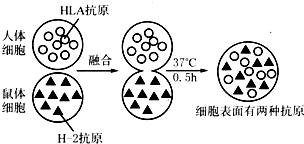
��1���˺���ϸ��Ĥ����Ŀ�ԭ���ڹ���Ĥ�ṹ�ĵ����ʣ����������ƣ���
��2���ں�ϸ����������ӫ��Ⱦ�Ϸֲ��Ķ�̬�仯�����������ϸ��Ĥ�ĵ����ʵȷ����ǿ����˶��ģ��ɴ˿���֤ʵ����ϸ��Ĥ�ṹ��ģ�͡�Ĥ���ʷ����ܹ��˶� �Ĺ۵��dz����ģ�
��3���ں�ϸ����������ӫ��Ⱦ�����վ��ȷֲ���ԭ���ǹ���Ĥ�ṹ����֬���Ӻ͵����ʷ��Ӵ����˶��ģ������ϸ��Ĥ�Ľṹ�ص��Ǿ���һ���������ԣ�
��4��������ں�ʵ����20�������½��У������ֱ��濹ԭ���ȷֲ���ʱ�佫����ӳ�����˵���滷���¶ȵĽ��ͣ�Ĥ�ϵ����ʷ��ӵ��˶����ʼ���������0��������40min������ϸ����Ȼ����һ�뷢��ɫӫ�⣬��һ�뷢��ɫӫ�⣮����һ����ĺ���������ϸ��Ĥ���������ص�ֻ�������˵������²������֣�
���� 1������Խ���ӵ�Ĥ�����ϵĵ����ʵ������������Խ�࣮
2��Ϊʹ�ˡ���ϸ��Ĥ�ں���һ�𣬱����ȥϸ��Ĥ������ʶ�����õ��ǵ��ף��������ںϣ�
3������ϸ��Ĥ����֬���Ӻ͵����ʷ��Ӷ��ǿ����˶��ģ�����Ĥ��������
4��Ĥ�������Ժ��¶��йأ�
��� �⣺��1������ϸ��Ĥ����Ŀ�ԭ���ڵ��������ʣ�
��2��ӫ��Ⱦ�ϱ�ǵĿ����뿹ԭ����ϣ���ӫ��Ⱦ�Ϸֲ��Ķ�̬�仯��˵�����ϸ��Ĥ�ĵ����ʷ��ӿ����˶����ɴ˿���֤ʵϸ��Ĥ�������ܹ��˶��Ĺ۵��dz����ģ�
��3��ϸ��Ĥ�Ľṹ�ص��Ǿ���һ���������ԣ�
��4������������Ĥ�ϵ����ʷ��Ӿ��ȷֲ���ʱ���ӳ���˵�������ʷ����˶��������¶ȵĽ���������ϸ��Ĥ���ӵ��˶����¶��йأ�����0��������40min������ϸ����Ȼ����һ�뷢��ɫӫ�⣬��һ�뷢��ɫӫ�⣬��˵��ϸ��Ĥ���������ص�ֻ�������˵������²������֣�
�ʴ�Ϊ��
��1��������
��2�������ʵ� Ĥ���ʷ����ܹ��˶�
��3������Ĥ�ṹ����֬���Ӻ͵����ʷ��Ӵ����˶��� һ����������
��4���滷���¶ȵĽ��ͣ�Ĥ�ϵ����ʷ��ӵ��˶����ʼ���
ϸ��Ĥ���������ص�ֻ�������˵������²�������
���� ���⿼����ϸ��Ĥ�Ľṹ�ͽṹ�ص㣬Ĥ�������Ժ��¶��йأ��¶Ƚ��ͣ������ʺ���֬���ӵ��˶��ٶȼ�����Ĥ�������ٶȼ�����

 ��ǰ����ϵ�д�
��ǰ����ϵ�д�| A�� | �����������ͬ | B�� | ������Ŀռ�ṹ��ͬ | ||
| C�� | �������Ȼ���C���ӵ�λ�ò�ͬ | D�� | �������ţ�R�����IJ�ͬ |
| A�� | ������ | B�� | ֬�� | C�� | ���� | D�� | ��ԭ |
| A�� | �������� | B�� | �������� | ||
| C�� | ���dz��������Ϊ�������� | D�� | ���Ǵ��������Ϊ�������� |
| A�� | �����塢�߶����塢������ | B�� | �߶����塢��������ϸ���� | ||
| C�� | �߶����塢��������ϸ��Ĥ | D�� | �����塢�߶����塢��������ϸ��Ĥ |
| A�� | ���췬��ϸ��Һ����Ȼ���зḻ�������Ǻ��ǣ���������������ԭ�Ǽ������������ | |
| B�� | ���ڼ��������ʵ�˫�����Լ�Ҫ�Ƚ�AҺBҺ��Ͼ��Ⱥ��ټ�����Ʒ�Թ��� | |
| C�� | ���������Ի�ԭ��ʱ��Ҫ�ȼ�������Լ���Һҡ�Ⱥ��ټ���Һ | |
| D�� | ����Լ���������Һˮԡ���5���ӽ�����ש��ɫ���� |
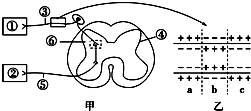
| A�� | ���۴����𣬵�̼��ݴ����ⲻ���� | |
| B�� | ��������ά�ܵ��̼���˲��Ĥ�����ɵķֲ�������ж���ͼ�е�a��cΪ�˷ܲ�λ | |
| C�� | ��ͼ������Ԫϸ��Ĥ�ھֲ�������������b����a��c | |
| D�� | �˷��ڷ��仡�еĴ�����˫��� |
| A�� | ���̲�������HIV�����˵�Tϸ��������ʹ����������������ȫ��ɥʧ | |
| B�� | ����ϵͳ��ά����̬�о�����Ҫ���� | |
| C�� | ����ĵ�����������Ҫ���������ٺ�����ϸ������ѪҺѭ�����ܰ�ѭ����ɵ� | |
| D�� | ϵͳ�Ժ���Ǵ������������ܲ�����ɵ� |