��Ŀ����
����Ŀ������Ϊ��Ҫ����ԭ�ϣ�������Ӧ���ڹ�ҵ���������ڲ�ͬ���������¿ɷ�������������Ӧ:
��ӦI��4NH3(g) +5O2(g)![]() 4NO(g) +6H2O(g) ��H=-905.0 kJ��mol��1
4NO(g) +6H2O(g) ��H=-905.0 kJ��mol��1
��Ӧ II��4NH3(g)+3O2(g)![]() 2N2(g) +6H2O(g) ��H=-1266.6kJ��mol��1
2N2(g) +6H2O(g) ��H=-1266.6kJ��mol��1
(1)д��NO�ֽ�����N2��O2���Ȼ�ѧ����ʽ��___________________________��
(2)��ӦI���ݻ��̶����ܱ������н��У������ڲ������ʵ����ʵ���Ũ�����±���
ʱ�� Ũ�� | c(NH3)/mol/Ll | c((O2)/mol/L | c(NO)/ mol/L |
�� 0 min | 0.8 | 1.6 | 0 |
�� 2 min | 0.3 | 0.975 | 0.5 |
�� 3 niin | 0.3 | 0.975 | 0.5 |
��4 min | 0.7 | 1.475 | 0.1 |
�ٷ�Ӧ�ӿ�ʼ����2minʱ��v(H2O)=__________________��
���ڵ�3minʱ���ı�ķ�Ӧ����������_________(��ѡ����ĸ)��
A.ʹ�ô��� B.�Сѹǿ C.�����¶� D.����O2��Ũ��
�۸÷�Ӧ�ﵽƽ��״̬�ı�־��___________(��ѡ����ĸ)��
A.�ں��º��ݵ������У����������ܶȲ��ٱ仯
B.��λʱ��������nmolNO��ͬʱ����nmolNH3
C.�ٷֺ���w(NH3)=w(NO)
D.��Ӧ����v(NH3):v(O2):v(NO):v(H2O)=4:5:4:6
(3)�������ݻ�Ϊ2L���ܱ������г���8molNH3(g)��6molO2(g),������ӦII�����������������䣬����ͬʱ���ڲ��c(N2)���¶ȵĹ�ϵ��ͼ��ʾ����T1���£�NH3��ƽ��ת����Ϊ___��
��ͼ��a��c�����Ӧ�������ڲ�ѹǿPa____Pc(�>������<����=��)

(4)������������������炙��ʡ�25��ʱ����֪NH3��H2O�ĵ��볣��Kb=1.8��10-5����������ڴ��¶��·���ˮ�ⷴӦ��ƽ�ⳣ��Kh=___________��
���𰸡� 2NO(g) ![]() N2(g)+O2(g) ��H=-180.8kJ/mol 0.375mol/(L��min) C B 60% > (5/9)��10-9(��5.6��10-10)
N2(g)+O2(g) ��H=-180.8kJ/mol 0.375mol/(L��min) C B 60% > (5/9)��10-9(��5.6��10-10)
��������(1)��֪����ӦI��4NH3(g) +5O2(g)![]() 4NO(g) +6H2O(g) ��H=-905.0 kJ��mol��1����Ӧ II : 4NH3(g)+3O2(g)
4NO(g) +6H2O(g) ��H=-905.0 kJ��mol��1����Ӧ II : 4NH3(g)+3O2(g)![]() 2N2(g) +6H2O(g) ��H=-1266.6kJ��mol��1�����ݸ�˹���ɿ�֪�� II-I����2�ɵ�2NO(g) =O2+N2(g) ����H=[(-1266.6 kJ��mol-1)-(-905.0 kJ��mol-1)]��2 = ��H= -180.8 kJ��mol-1��NO�ֽ�����N2��O2���Ȼ�ѧ����ʽΪ2NO(g)
2N2(g) +6H2O(g) ��H=-1266.6kJ��mol��1�����ݸ�˹���ɿ�֪�� II-I����2�ɵ�2NO(g) =O2+N2(g) ����H=[(-1266.6 kJ��mol-1)-(-905.0 kJ��mol-1)]��2 = ��H= -180.8 kJ��mol-1��NO�ֽ�����N2��O2���Ȼ�ѧ����ʽΪ2NO(g) ![]() N2(g) +O2(g) ��H= -180.8 kJ��mol-1��(2) �ٷ�Ӧ�ӿ�ʼ����2minʱ��v(H2O)=
N2(g) +O2(g) ��H= -180.8 kJ��mol-1��(2) �ٷ�Ӧ�ӿ�ʼ����2minʱ��v(H2O)=![]() v(NO)=
v(NO)=![]() 0.375mol/(L��min)�����ڵ�3min��4min���������ݿ�֪��Ӧ���������淽���ƶ�����Ϊ�÷�ӦΪ�����������ķ��ȷ�Ӧ����A.ʹ�ô�������ʹƽ���ƶ���ѡ��A��ѡ��B.�Сѹǿƽ��������������������Ӧ�����ƶ��������ϣ�ѡ��B��ѡ��C.�����¶�ƽ�������ȷ�Ӧ���淽���ƶ��������ϣ�ѡ��C��ѡ��D.����O2��Ũ��ƽ��������Ӧ�����ƶ��������ϣ�ѡ��D��ѡ����ѡC����A�����������ܶȲ��ٸı��״̬ �����������£���������������䣬����������ܶ�һֱ���䣬������Ϊ�ﵽƽ��״̬�ı�־��ѡ��A����B����λʱ��������nmolNO��ͬʱ����nmolNH3���������淴Ӧ������ȣ�����Ϊ�ﵽƽ��״̬�ı�־��ѡ��B��ȷ��C���ðٷֺ���w(NH3)=w(NO) ��һ����ƽ��״̬�����Բ�����Ϊ�ﵽƽ��״̬�ı�־��ѡ��C����D����NH3��O2��NO��H2O�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���ʵı�Ϊ4:5:4:6��״̬����һ����ƽ��״̬�����Բ�����Ϊ�ﵽƽ��״̬�ı�־��ѡ��D����ѡB��(3)��T1���£�c(N2)=1.2mol/L�������ɵĵ���Ϊ1.2mol/L
0.375mol/(L��min)�����ڵ�3min��4min���������ݿ�֪��Ӧ���������淽���ƶ�����Ϊ�÷�ӦΪ�����������ķ��ȷ�Ӧ����A.ʹ�ô�������ʹƽ���ƶ���ѡ��A��ѡ��B.�Сѹǿƽ��������������������Ӧ�����ƶ��������ϣ�ѡ��B��ѡ��C.�����¶�ƽ�������ȷ�Ӧ���淽���ƶ��������ϣ�ѡ��C��ѡ��D.����O2��Ũ��ƽ��������Ӧ�����ƶ��������ϣ�ѡ��D��ѡ����ѡC����A�����������ܶȲ��ٸı��״̬ �����������£���������������䣬����������ܶ�һֱ���䣬������Ϊ�ﵽƽ��״̬�ı�־��ѡ��A����B����λʱ��������nmolNO��ͬʱ����nmolNH3���������淴Ӧ������ȣ�����Ϊ�ﵽƽ��״̬�ı�־��ѡ��B��ȷ��C���ðٷֺ���w(NH3)=w(NO) ��һ����ƽ��״̬�����Բ�����Ϊ�ﵽƽ��״̬�ı�־��ѡ��C����D����NH3��O2��NO��H2O�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ���ʵı�Ϊ4:5:4:6��״̬����һ����ƽ��״̬�����Բ�����Ϊ�ﵽƽ��״̬�ı�־��ѡ��D����ѡB��(3)��T1���£�c(N2)=1.2mol/L�������ɵĵ���Ϊ1.2mol/L![]() 2L=2.4mol�����ݷ�Ӧ4NH3(g)+3O2(g)
2L=2.4mol�����ݷ�Ӧ4NH3(g)+3O2(g)![]() 2N2(g) +6H2O(g)��֪���ĵİ���Ϊ4.8mol����ת����Ϊ
2N2(g) +6H2O(g)��֪���ĵİ���Ϊ4.8mol����ת����Ϊ![]() ����c���¶Ƚϸ�ƽ�������ƶ�������������ʵ������٣�ѹǿ��С����ͼ��a��c�����Ӧ�������ڲ�ѹǿPa>Pc��(4) 25��ʱ����֪NH3��H2O�ĵ��볣��Kb=1.8��10-5����NH4++H2O
����c���¶Ƚϸ�ƽ�������ƶ�������������ʵ������٣�ѹǿ��С����ͼ��a��c�����Ӧ�������ڲ�ѹǿPa>Pc��(4) 25��ʱ����֪NH3��H2O�ĵ��볣��Kb=1.8��10-5����NH4++H2O![]() NH3��H2O+H+��Kh=
NH3��H2O+H+��Kh=![]()

����Ŀ���ں����ܱ�������ͨ��A��B�������壬��һ�������·�����Ӧ��
2A(g)��B(g)![]() 2C(g) ��H>0��
2C(g) ��H>0��
�ﵽƽ��ı�һ������(x)��������(y)һ������ͼ�����ߵ���(����)
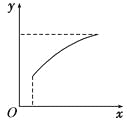
ѡ�� | x | y |
A | ��ͨ��A | B��ת���� |
B | ������� | A��������� |
C | ѹǿ | �������������ʵ��� |
D | �¶� | �������������ʵ��� |
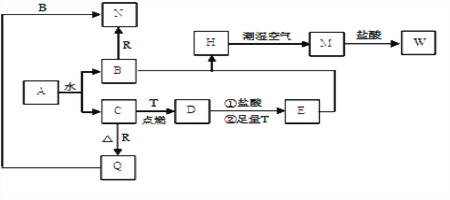
����Ŀ����������Ҫ�ɷ�ΪFeO��Cr2O3������SiO2��Al2O3�����ʡ���ҵ���ø������Ʊ��췯�ƾ���(Na2Cr2O7)��������ͼ��ʾ:

(1)����ٵ���Ҫ��ӦΪFeO��Cr2O3+O2+NaOH![]() Na2CrO4+NaFeO2+H2O���÷�Ӧ��ƽ��FeO��Cr2O3��O2��ϵ����Ϊ___________���ò������������н������գ����ô��������ϵ���______________(����)��
Na2CrO4+NaFeO2+H2O���÷�Ӧ��ƽ��FeO��Cr2O3��O2��ϵ����Ϊ___________���ò������������н������գ����ô��������ϵ���______________(����)��
A.�� B.������ C.ʯӢ D.�մ�
(2)���ۢ����շ�Ӧ��������Ҫ������NaOH������״̬����Ӧ���ʲżӿ죬��ԭ����____________________________________________________��
(3)�������NaFeO2��ǿ��ˮ����������������������Ӧ�Ļ�ѧ����ʽΪ________________��
(4)�������Һ1�ֱ���130������1Сʱ��������ȴ����ͬ�¶��½ᾧ�����¹��ˣ�����ʵ���������±����������ݷ���������۵���ѽᾧ�¶�Ϊ___________�档
�ᾧ�¶�/�� | Na2CrO4�־��и����ʺ���/% | |||
Na2CrO4 4H2O | NaOH | NaAlO2 | Na2SiO3 | |
30 | 52.45 | 29.79 | 8.69 | 12.21 |
40 | 68.81 | 20. 49 | 8.46 | 10.84 |
50 | 60.26 | 27. 96 | 10.36 | 9.32 |
60 | 50.74 | 29.66 | 10.40 | 12.25 |
70 | 46.77 | 33.06 | 8.10 | 6.48 |
(5)�����������3�ijɷ���_____________(д��ѧʽ)��
(6)���������и�Ԫ����ȫת��Ϊ�췯�ƾ��壬��ø������и�Ԫ�ص���������Ϊ____________(�ú�m1��m2�Ĵ���ʽ��ʾ)��