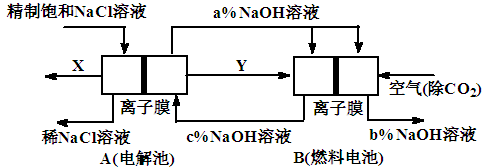
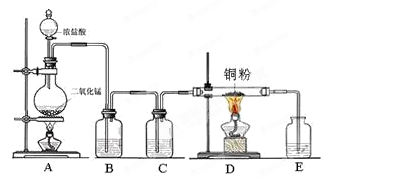
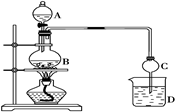
��Ŀ����
��10�֣�CO��һ����ɫ����ζ�Ҳ�����ˮ���ж����壬���Ʒ��У�
��1��ʵ���ҿ��ü����Ũ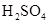 ������ȡCO��
������ȡCO��
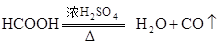
a�������Ũ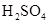 Ӧ������ϣ�Ũ
Ӧ������ϣ�Ũ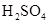 ��ʲô����?
��ʲô����?
b�����ʵ��ԭ��ѡ��������Ҫ�������������ܳ��⣩��
c�������ռ�CO����?
��2��ʵ���һ����ò��ᾧ����Ũ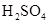 ������ȡCO��
������ȡCO��
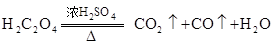
���ʣ��������ܵõ����� ��CO����?
��CO����?
��3����ҵ���ý�̿��ˮ�ڸ����·�����Ӧ��ȡCO���仯ѧ����ʽΪ��____________
____���ڴ������£�C��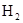 �Ļ�ԭ��˭ǿ?
�Ļ�ԭ��˭ǿ?
��1��ʵ���ҿ��ü����Ũ
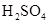 ������ȡCO��
������ȡCO��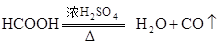
a�������Ũ
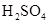 Ӧ������ϣ�Ũ
Ӧ������ϣ�Ũ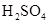 ��ʲô����?
��ʲô����?b�����ʵ��ԭ��ѡ��������Ҫ�������������ܳ��⣩��
c�������ռ�CO����?
��2��ʵ���һ����ò��ᾧ����Ũ
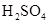 ������ȡCO��
������ȡCO��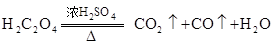
���ʣ��������ܵõ�����
 ��CO����?
��CO����?��3����ҵ���ý�̿��ˮ�ڸ����·�����Ӧ��ȡCO���仯ѧ����ʽΪ��____________
____���ڴ������£�C��
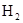 �Ļ�ԭ��˭ǿ?
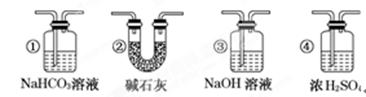
�Ļ�ԭ��˭ǿ?��1��a������������ȵ�ŨH2SO4�У���ˮ���ã���b����Һ©����װ���ᣩ��Բ����ƿ��װŨH2SO4�����ƾ��ơ�����ܡ�����ƿ����c������ˮ���ռ���
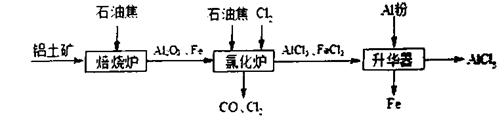
������2��ͨ����ʯ�һ�NaOH��Һ������3��C��H2O(g)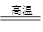 H2��CO ��ԭ��C��H2
H2��CO ��ԭ��C��H2
������2��ͨ����ʯ�һ�NaOH��Һ������3��C��H2O(g)
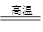 H2��CO ��ԭ��C��H2
H2��CO ��ԭ��C��H2��1��a Ũ���������ˮ�ԣ��Ҽ�����ܶ�С��Ũ����ģ�����Ҫ�õ�CO��Ӧ�ý���������ȵ�ŨH2SO4�С�
b ��ʵ��ԭ����Һ���Һ��֮�������ȡ����ģ�������Ҫ�������з�Һ©����װ���ᣩ��Բ����ƿ��װŨH2SO4�����ƾ��ơ�����ܡ�����ƿ��
c CO������ˮ�����ܶȺͿ����ܽӽ�������Ӧ��������ˮ���ռ���
��2��CO2����������������ڼ��У�����Ҫ��ȥCO�е�CO2�����Խ������ͨ����ʯ�һ�NaOH��Һ��
��3�������£�̼��ˮ������Ӧ����������������CO�����Է���ʽΪC��H2O(g)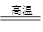 H2��CO������������ԭ��Ӧ�У���ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ŀ��жϣ���ԭ����C��H2.
H2��CO������������ԭ��Ӧ�У���ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ŀ��жϣ���ԭ����C��H2.
b ��ʵ��ԭ����Һ���Һ��֮�������ȡ����ģ�������Ҫ�������з�Һ©����װ���ᣩ��Բ����ƿ��װŨH2SO4�����ƾ��ơ�����ܡ�����ƿ��
c CO������ˮ�����ܶȺͿ����ܽӽ�������Ӧ��������ˮ���ռ���
��2��CO2����������������ڼ��У�����Ҫ��ȥCO�е�CO2�����Խ������ͨ����ʯ�һ�NaOH��Һ��
��3�������£�̼��ˮ������Ӧ����������������CO�����Է���ʽΪC��H2O(g)
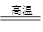 H2��CO������������ԭ��Ӧ�У���ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ŀ��жϣ���ԭ����C��H2.
H2��CO������������ԭ��Ӧ�У���ԭ���Ļ�ԭ�Դ��ڻ�ԭ����Ŀ��жϣ���ԭ����C��H2.
��ϰ��ϵ�д�
�����Ŀ
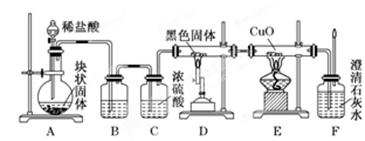
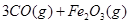

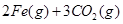 ����Ӧ�ڵ�ƽ�ⳣ���ı���ʽΪK= ��
����Ӧ�ڵ�ƽ�ⳣ���ı���ʽΪK= ��