题目内容
1g H2燃烧生成液态水放出142.9kJ的热量,下列热化学方程式正确的是
A.2H2(g)+ O 2(g) = 2H2O(1)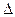 H = —142.9kJ·molˉl H = —142.9kJ·molˉl |
B.2H2(g)+ O 2(g) = 2H2O(1)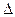 H = —571.6kJ·molˉl H = —571.6kJ·molˉl |
C.2H2+O2 = 2H2O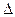 H = —571.6lkJ·molˉl H = —571.6lkJ·molˉl |
D.H2(g)+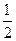 O 2(g) = H2O(1) O 2(g) = H2O(1)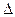 H = +285.8kJ·molˉl H = +285.8kJ·molˉl |
B
略

练习册系列答案
相关题目
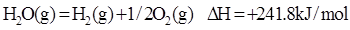
 ??cC(g)+dD(g),取a mol A和b mol B置于V L密闭容器中,2 min后,测得容器中A的浓度为x mol·L-1,以物质C的浓度变化来表示这段时间内反应的平均速率应为__________ __。
??cC(g)+dD(g),取a mol A和b mol B置于V L密闭容器中,2 min后,测得容器中A的浓度为x mol·L-1,以物质C的浓度变化来表示这段时间内反应的平均速率应为__________ __。
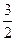 O2(g) ="=" CO2(g) + 2H2O(l);△H=-726.4kJ/mol
O2(g) ="=" CO2(g) + 2H2O(l);△H=-726.4kJ/mol  SO3(g);△H=-98.3KJ·mol-1
SO3(g);△H=-98.3KJ·mol-1