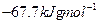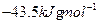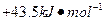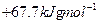��Ŀ����
(9��)(1)1 mol��̬�����Ӻ�1 mol��̬�����ӽ������1 mol�Ȼ��ƾ����ͷų�������Ϊ�Ȼ��ƾ���ľ����ܡ�
�������Ȼ�ѧ����ʽ�У���ֱ�ӱ�ʾ���Ȼ��ƾ��徧���ܵ���____________��
E.Cl2(g)�D��Cl(g)����Q4
F��Cl(g)��e���D��Cl��(g)����Q5
��д����Q1���Q����Q2����Q3����Q4����Q5֮��Ĺ�ϵʽ________________________________________________________________________
(2)���淴Ӧ��aA(g)��bB(g) ??cC(g)��dD(g)��ȡa mol A��b mol B����V L�ܱ������У�2 min���������A��Ũ��Ϊx mol��L��1��������C��Ũ�ȱ仯����ʾ���ʱ���ڷ�Ӧ��ƽ������ӦΪ__________ __��
??cC(g)��dD(g)��ȡa mol A��b mol B����V L�ܱ������У�2 min���������A��Ũ��Ϊx mol��L��1��������C��Ũ�ȱ仯����ʾ���ʱ���ڷ�Ӧ��ƽ������ӦΪ__________ __��
�������Ȼ�ѧ����ʽ�У���ֱ�ӱ�ʾ���Ȼ��ƾ��徧���ܵ���____________��
| A��Na��(g)��Cl��(g)�D��NaCl(s)����Q |
| B��Na(s)��Cl2(g)�D��NaCl(s)����Q1 |
| C��Na(s)�D��Na(g)����Q2 |
| D��Na(g)��e���D��Na��(g)����Q3 |
F��Cl(g)��e���D��Cl��(g)����Q5
��д����Q1���Q����Q2����Q3����Q4����Q5֮��Ĺ�ϵʽ________________________________________________________________________
(2)���淴Ӧ��aA(g)��bB(g)
 ??cC(g)��dD(g)��ȡa mol A��b mol B����V L�ܱ������У�2 min���������A��Ũ��Ϊx mol��L��1��������C��Ũ�ȱ仯����ʾ���ʱ���ڷ�Ӧ��ƽ������ӦΪ__________ __��
??cC(g)��dD(g)��ȡa mol A��b mol B����V L�ܱ������У�2 min���������A��Ũ��Ϊx mol��L��1��������C��Ũ�ȱ仯����ʾ���ʱ���ڷ�Ӧ��ƽ������ӦΪ__________ __����1�� ��A ���ڡ�Q1����Q����Q2����Q3����Q4����Q5
(2)��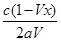 mol/(L��min)
mol/(L��min)
(2)��
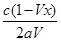 mol/(L��min)
mol/(L��min)�Ţ��ɾ����ܵĶ��壬��̬�����ӡ���̬�����ӡ�����1 mol�Ȼ��ƾ��塢�ͷų������ܣ�����A��ȷ��
�ڡ�Q1����Q����Q2����Q3����Q4����Q5
��
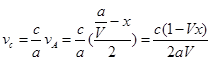 mol/(L��min)
mol/(L��min)
��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
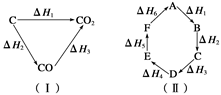
 Si��s����4HCl��g�����÷�Ӧ�ķ�Ӧ
Si��s����4HCl��g�����÷�Ӧ�ķ�Ӧ �ȡ�HΪ ��������
�ȡ�HΪ ��������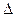 H=��570 kJ��mol-1��
H=��570 kJ��mol-1�� 2O (1)����H��-285.8 kJ��mol-1
2O (1)����H��-285.8 kJ��mol-1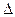 H = ��142.9kJ��mol��l
H = ��142.9kJ��mol��l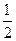 O 2(g) = H2O(1)
O 2(g) = H2O(1)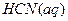 ��
��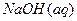 ��Ӧ��
��Ӧ��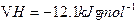 ��
��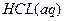 ��
��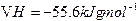 ����
����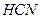 ��ˮ��Һ�е����
��ˮ��Һ�е���� ����
����